Abstract
In this work, we prepared different alkyl acrylates by esterifying acrylic acid with different alcohols (decanol, dodecanol, hexadecanol and octadecanol). Anilimide was then produced by the reaction of aniline with maleic anhydride. Different teropolymers were prepared by polymerization reaction of anilimide, different alkyl acrylate esters and olefins in different ratios. The thermal stability of the prepared terpolymers was measured by thermal gravimetric analysis which demonstrated a high thermal stability. The polymers were degraded above 500 °C. The rheology behavior shows shear-thinning, it approaches the ideal Newtonian behavior in case of polymer (C). The prepared terpolymers succeeded in raising the viscosity index of oil to 118 in case of polymer (C) and decreasing the pour point of oil to -12 in case of polymer (E).
Similar content being viewed by others
Introduction
Lubrication can be defined as the application of some materials between two objects moving relative to each other to allow smooth operation as much as necessary. Because the lubricants spent their service-life or polluted with foreign materials cannot serve their function well, they have to be periodically replaced or oiled. Main purposes of lubrication are (1) to prevent wear and premature fatigue by forming the lubrication film on the surface of load transferring parts to prevent contacts between metals, (2) To enhance the favorable driving characteristics, such as low friction, (3) To prevent overheating of bearings and to prevent lubricant s own deterioration by radiating the generated heat to outside. It works particularly well if the circulation lubrication method is adopted, (4) To prevent foreign material penetration, rust, and corrosion [1].Lubricants can be largely divided into two groups, namely mineral base oil lubricants and synthetic lubricants. When selecting a lubricant, the most important factors to be considered is its viscosity. If its viscosity is too low at its operating temperature, oil film cannot be sufficiently formed, causing wear and/or burning-and-sticking. And, if it is too high, its viscosity resistance becomes higher, causing temperature/friction rise and subsequent abnormal power loss. In general, lubricants with low viscosity are used at high speed and low load, and ones with high viscosity are used at low speed and high load. To develop and improve the properties and performance of lube oil, we use lubricating oil additives [2, 3]. Modern lubricants, especially motors oils, contain polymeric additives that modify the viscosity characteristics of the lubricants. These additives are called viscosity modifiers or viscosity index improvers. The viscosity index improvers impact the oil behavior as far as flow characteristics are concerned [4, 5]. All mineral derived base stocks used in lubricants contain waxy hydrocarbons that come out of solution when temperature decreases. They can form a three-dimensional wax crystal network that can totally immobilize the oil. Such waxiness has long been recognized in mineral oils and formulated fluids and most familiarly described by pour point [6]. The dewaxing process is used to lower the pour point of the oil. In general, inhibition of wax crystallization has been considered to occur in the presence of pour point depressant (PPD) by nucleation, co-crystallization or adsorption. It is generally believed that the PPD function by disrupting or preventing the formation of three-dimensional wax networks, leaving the amount of crystalline wax unaffected [7]. The present work aims to the preparation of different terpolymers by the reaction of different alkyl acrylates with anilimide and octene in different ratios and evaluation of the prepared terpolymers as viscosity index improvers and pour point depressants for lubricating oils.
Experimental
Esterification of acrylic acid
Alkyl acrylate is prepared by the reaction of 1 mol of acrylic acid with 1 mol of different types of alcohols (decanol, dodecanol, hexadecanol and octadecanol). The reactions were carried out with xylene as a solvent and in the presence of 0.5% of p-toluene sulfonic acid as a catalyst and 0.25% of hydroquinone to inhibit the reaction on the double bond of acrylic acid. The reactants, were heated gradually to 140 °C—160 °C, using a well-controlled thermostat to give products decyl acrylate (A1), dodecyl acrylate (A2) hexadecyl acrylate (A3), and octadecyl acrylate (A4) [8].
Preparation of anilimide
One mole of aniline dissolving in 50 ml of T.H.F. were added to maleic anhydride dropwise over a period of 2 h at room temperature. The mixture was stirred for 7 h. at room temperature then the temperature was raised gradually till 70 °C. Then 2 gm. of sodium acetate was added, and 3 mol of acetic anhydride were added dropwise. The reaction was kept stirred for 16 h. The product was washed with iced water and filtered followed by washing with a solution of 5% sodium bicarbonate to neutralize the rest of maleimic acid followed by washing with iced water and filtration as shown in Scheme 1 [9].
Preparation of terpolymers with changing alkyl acrylate chain length
Three polymers were prepared by free radical terpolymerization of different ratios of prepared imide, lower and higher prepared esters (A1, A2 andA4) and octene. The polymerization was carried out in 4-necked flask equipped with a stirrer, efficient condenser, thermometer, and an inlet for the introduction of nitrogen. The reactants (imide, octene, and ester) were mixed with toluene as a solvent and benzoyl peroxide as a catalyst. The reaction temperature was maintained at 60 °C–80 °C for 8 h. When the reaction was completed the product was stirred with high speed in cold methanol to separate the polymer followed by filtration and drying [10]. The monomer ratios of first group of prepared terpolymers were illustrated in Table 1
Preparation of terpolymers with changing monomer ratio
Polymeric additives were prepared by free radical terpolymerization of different ratios of prepared imide, prepared ester (A3) and octene. The polymerization was carried out in 4-necked flask in the same last manner. The monomer ratios of second group of prepared terpolymers were illustrated in Table 2
Infrared spectroscopic analysis
(IR) spectra of the synthesized esters were measured by FTIR Spectrometer Model Type Mattson Infinity series Bench top 961 for the purified esters [11].
Determination the molecular weight of the prepared terpolymers
The molecular weights of different prepared terpolymers were determined by Gel Permeation Chromatography (GPC) waters model 510 using polystyrene standard, Ultra-styragel column, and tetrahydrofuran as an eluent [12].
H1-NMR spectroscopic analysis
The structure of the synthesized additives were followed up by Proton Nuclear Magnetic Resonance (H1-NMR) spectra using a 300 MHz Varian NMR 300 spectrometer using (DMSO-d6) as a solvent.
Evaluation of the prepared compounds as viscosity index improver for lube oil
The evaluation was carried out using the ASTM D2270-87 method for measuring the viscosity index (VI).
Evaluation of the prepared compounds as pour point depressants for lube oil
This evaluation was carried out using the ASTM-D 98–87 for measuring the pour point (PP) on cryostat apparatus for pour point measurement [13].
Results and discussion
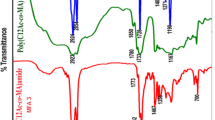
Polymers used as viscosity modifiers in oil have three critical attributes: improved viscosity index, enhanced thickening efficiency, and appropriate shear stability, which results in enhancing the lubricant performance and extending the equipment life. The chemical structures of the prepared ester (A3) and prepared imide were confirmed by IR spectrum and molecular weight. IR spectra Figs. 1, 2 are analyzed in Tables 3, 4, Fig. 1 show the disappearance of the strong band of both acid and alcohol at 3500 cm−1 due to product well purification indicates that all acids and alcohols are consumed in ester formation. Figure 2 show the disappearance of two peaks at 1818 and 1750 cm−1 that represents the absence of anhydride carbonyl group and their replacement with –C = O imide peak at 1720 cm−1 ensure transformation of all anhydrides into imides, This indicates that the esterification and amination reactions were completed successfully and confirms the formation of the desired compounds. The mean molecular weights of copolymers are given in Table 5,which indicates that the molecular weight of terpolymer increases with increasing the percentage of acrylates in the polymer reaching 42001 in the case of (A3 3) containing 70% hexadecyl acrylate, but when hexadecyl acrylate percentage increases to 75% the molecular weight of polymer decreases to 36926.The molecular weight of polymer also increases by increasing the alkyl acrylate chain length till reaching 42001 in the case of (A3 3) containing hexadecyl acrylate in its composition, then the molecular weight decreases reaching 10693 in the case of (A4 3) containing octadecyl acrylate in its composition, this may be due to increasing the alkyl chain length, making a coil that makes steric hindrance around the function groups which hinder polymerization [14].The polymer structure was confirmed using (H1NMR). The spectrum is shown in Fig. 3 and was analyzed in Table 6, which proved the chemical structure of the terpolymer as shown in Fig. 4.
Thermal stability of the prepared terpolymer (A3 1) was indicated using thermo gravimetric analysis (TGA) from Fig. 5 it was found that the polymer degrade completely at temperature above 400 °C, which indicates that prepared terpolymers have high thermal stability [15]. As shown in Fig. 6, the thermogravimetric curve of the prepared terpolymers A1 3 shows two steps of the weight loss. The primary degradation occurs at 100 °C with weight loss of 38.5%. This can be attributed to the evaporation of intra and intermolecular moisture. The major degradation occurs at 390 °C with weight loss of 62.9%, which is corresponding to degradation of polymer backbone. According to A2 3 curve the primary degradation occurs at 75 °C with weight loss of 9.35%. The second degradation occurs at 200 °C with weight loss of 12.66%. The third degradation occurs at 310 °C with weight loss of 69.8%. The last degradation occurs at 490 °C with weight loss of 6.7%. According to A4 3 curve the primary degradation occurs at 125 °C with weight loss of 18.7%. The second and major degradation occurs at 300 °C with weight loss of 66.8%. The third degradation occurs at 450 °C with weight loss of 14%.
Evaluation of the prepared terpolymers as viscosity index improvers for lube oil
Effect of change in alkyl acrylate chain length
The soluble terpolymers with change in alkyl acrylate chain length (A1 3, A2 3, A3 3 and A4 3) were tested for their effectiveness as viscosity index improvers for base oil (SAE 30).The results were shown in Table 7.From the results, it was found that the viscosity index of the lube oil increases by increasing the additive concentration, the viscosity index of the oil also increases by increasing the alkyl acrylate chain length in the polymer reaching 118 in case of polymer (A3 3), which contains hexadecyl acrylate in its composition but it decreases after that in the case of polymer(A4 3), which contains octadecyl acrylate in its composition Fig. 7.This may be due to forming a coil which hinders expansion with increasing temperature [16].
Effect of change in alkyl acrylate percentage
The soluble terpolymers with change in hexadecyl acrylate percentage (A3 1, A3 2, A3 3 and A3 4) were tested for their effectiveness as viscosity index improvers. The results were shown in Table 8, it was found that the viscosity index of the lube oil increases by increasing the additive concentration, the viscosity index of the oil also increases by increasing the percentage of acrylate in the polymer.Polymer (A3 3) with composition: Anilimide (5%) + hexadecyl acrylate (70%) + octene (25%) with concentration 3% gives better viscosity index results 118.
Evaluation of the prepared terpolymers as pour point depressant for lube oil
Effect of change of alkyl acrylate chain length in polymer
The effect of alkyl acrylate chain length on P.P. for lube oil doped with (A1 3, A2 3, A3 3 and A4 3) additives was tested and shown in Fig. 8 it is clear that the pour point decreases with decreasing the alkyl chain length. Polymer (A1 3) with C10 alkyl chain length give better pour point results -12 with composition: Anilimide (5%) + decyl acrylate (70%) + octene (25%). This is because the increase in chain length may cause extra steric hindrance, which results in the formation of lower molecular weight terpolymer.
Effect of change of hexadecyl acrylate percentage in polymer
The effect of alkyl acrylate percentage on P.P. for lube oil doped with (A3 1, A3 2, A3 3 and A3 4) additives was tested and shown in Fig. 9, which shows that there was no noticeable change in pour point due to the approaching of the alkyl acrylate concentration in the different terpolymers (A3 1, A3 2, A3 3 and A3 4).
Effect of pour point depressants on wax crystal modification
Pour point depressants act through surface adsorption onto the wax crystals. The resulting surface layer of pour point depressant inhibits the growth of the wax crystals and their capacity to adsorb oil and form gels. Photoanalysis confirms other standard flow tests that evaluate the cold flow properties of untreated/treated lube oil through wax crystallization behavior [17]. It is applied here in for assessing the action of the previously prepared terpolymers flow additives as wax inhibitor/pour point depressant through wax modification according to their type and concentration. Photomicrographs illustrated in Fig. 10 showed variant wax morphology for untreated lube oil showed large cyclic like crystals which on treatment with terpolymers (A1 3,A2 3) using 0.25% concentration by weight, show a significant reduction of wax crystal size and formation of abundant number of fine dispersed crystals. The dark droplet in Fig. 10 shows the accumulation of wax crystals while after using additives the photo became more clear because of the absence of accumulation of fine crystals and the change in morphology structure of crystal network.
Study the rheological properties of the prepared additives on lube oil
As shown in Fig. 11, the prepared samples show shear-thinning behavior. Their viscosity decreases when the shear rate increases. But it is noticed that the rate of decrease in viscosity by increasing shear rate reduces by increasing the percentage of hexdecyl acrylate in the terpolymer to approach the ideal Newtonian behavior in case of polymer (A3 3) in which, the viscosity of Newtonian fluids will remain a constant, no matter how fast they are forced to flow through a pipe or channel (i.e., viscosity is independent on the rate of shear) [18]. These conditions give long life to the lube oil and it is unnecessary to be changed.
Conclusion
All the prepared polymers are soluble in lube oil. Prepared additives have high thermal stability. All the prepared polymers are effective as viscosity index improver & pour point depressant. The viscosity index of the lubricant increases with increasing prepared additive concentration. The viscosity index of the lubricant increases with increasing the percentage of acrylate in the polymer to 70% but then decreases in the case of increasing the acrylate percentage to 75%. The viscosity index of the lubricant increases with increasing the alkyl chain length of acrylate in the polymer till hexdecyl acrylate. The pour point decreases with decreasing the molecular weight of the prepared additive. Our samples show shear-thinning behavior, their viscosity decreases when the shear rate increases. It is noticed that the rate of decrease in viscosity by increasing shear rate reduces by increasing the percentage of hexdecyl acrylate in the terpolymer.
References
Nassar AM, Ahmed NS, El-Shazly RI, Abdel Menem YK (2017) Preparation and evaluation of mixtures of sulfonate and phenate as lube oil additives. Int J Indust Chem: 1–13
Rudnick LR (2017) Lubricant additives: chemistry and applications. 3rd edn. CRC Press Taylor & Francis Group. https://doi.org/10.1201/9781315120621
Jun K (2017) Molecular science of lubricant additives. Appl Sci 7:445
Ashlie M, Uma S, Michelle L (2018) Review of viscosity modifier lubricant additives. Tribol Lett 66:2
Juliette L, Claire N, Jean-Jacques R, Vincent L, Sylvain C (2020) Synthesis of alkyl sulfur-functionalized oleic acid-based polymethacrylates and their application as viscosity index improvers in a mineral paraffinic lube oil. J Am Oil Chem Soc 97(3):309–318
Kamal RS, Ahmed NS, Nasser AM (2017) Synthesis and evaluation of multifunctional lube oil additives. Noor Publis, OMN
Kamal RS (2019) Types, examples and working mechanism of rheo-improvers lubricating oil additives. PPEJ 2:5
Nassar AM, Ahmed NS, Kamal RS, Abdel A-AA, El-NagdyAzim EI (2005) Preparation and evaluation of acrylate polymers as viscosity index improvers for lube oil. Petrol Sci Tech 23:537–546
Sheng-Huei H, Chia-Yin T, Yu-Ruei K (2015) Synthesis and characterization of novel electrochromic poly(amide-imide)s with N, N’-di(4-methoxyphenyl)-N, N'-diphenyl-p-phenylenediamine units. RSC Adv 5:93591–93606
Abdel A-A, Azim EI, El-Nagdy EI, Nassar Ahmed AM, Kamal RS (2006) Preparation and evaluation of acrylate polymers as pour point depressants for lube oil. Pet Sci Technol 24:887–894
Rabelo SN, Ferraz VP, Oliveira LS, Franca AS (2015) FTIR analysis for quantification of fatty acid methyl esters in biodiesel produced by microwave-assisted transesterification. Int J Indust Chem 6:12
Jing Wu, Li M, Zhang A (2018) Synthesis and characterization of SSS/HAM/AA terpolymer as a fluid loss additive for oil well cement. J Appl Polym Sci 135:46266
Ahmed NS, Nassar AM, Yasser K, el Menem A, Reham El- shazly I (2014) Preparation, characterization and evaluation of some metallic lube oil additives. IOSR-J Appl Chem 7(12):56–67
Sandeep C (2015) Acrylic acid and methacrylic acid based hydrogels-A review. Der Chemica Sinica 6(1):61–72
Rahangdalele SS, Gurnule WB (2012) Synthesis and thermogravimetric analysis of terpolymer resins derived from salicylaldoxime, melamine and formaldehyde. Der Pharma Chemica 4(5):1836–1846
Rudnick LR (2020) Synthetic, mineral oils and bio-based lubricants: chemistry and technology. 3rd edn. CRC Press Taylor & Francis Group. ISBN 9781138068216
Mohamad SA, Ahmed NS, Hassanein SM, Rashad AM (2012) Investigation of polyacrylates copolymers as lube oil viscosity index improvers. J Petrol Sci Eng 100:173–177
José ML, López ER, Gómez MG, Vilar SY, Yolanda P, José R, Gonçalves DEP, Seabra JHO, Josefa F (2020) Tribological behavior of nanolubricants based on coated magnetic nanoparticles and trimethylolpropane trioleate base oil. Nanomaterials 10(4):683
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-shazly, R.I., Kamal, R.S., Nassar, A.M. et al. “The behavior of some terpolymers as lubricating oil additives”. Appl Petrochem Res 10, 115–123 (2020). https://doi.org/10.1007/s13203-020-00250-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-020-00250-y