Abstract
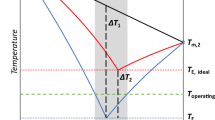
A theoretical and experimental study is performed for liquid–solid phase equilibria in the two-component diphenyl oxide–n-hexadecane system. The results show that this system is eutectic. Two means are chosen for the theoretical study of the system: the Schröder–Le Chatelier equations and the original UNIFAC system. Experimental studies are made via differential thermal analysis on a DSC unit. Results from the theoretical and experimental studies are presented in the form T–x diagrams of the system. The theoretical and experimental data are compared to one another. Each way of calculating is assessed, and recommendations are made for their application. The eutectic coordinates (melting point and composition) are determined experimentally. The following values are determined experimentally for a eutectic melt: the enthalpy of melting; density at 25°C; kinematic viscosity in the temperature range of 25 to 50°C; flash point in an open cup; boiling point, and refractive index in the temperature range of 25 to 40°C. The composition of the eutectic melt can be used in industry as a heat transfer fluid.



Similar content being viewed by others
REFERENCES
S. Z. Kagan and A. V. Chechetkin, Organic High Temperature Heat Transfer Fluids and Their Application in Industry (Gos. Nauch.-Tekh. Izd. Khim. Liter., Moscow, 1951) [in Russian].
A. V. Chechetkin, High Temperature Heat Carriers (Energiya, Moscow, 1971) [in Russian].
Sheng Fang, Xiao-Bo Zuo, Xue-Jiao Xu, and Da-Hai Ren, J. Chem. Thermodyn. 68, 281 (2014).
S. Sharma and M. Makavana, Fluid Phase Equilib. 375, 219 (2014).
P. Rice and S. Teja Amyn, J. Chem. Eng. Data 25, 346 (1980).
Lijiao Yu, Hong Dong, Chuan Wu, and Yindi Zhang, J. Chem. Thermodyn. 72, 139 (2014).
Yindi Zhang, Hong Dong, Yan Yue, and Chuan Wu, J. Chem. Thermodyn. 57, 114 (2013).
I. K. Garkushin, A. V. Kolyado, and I. G. Yakovlev, Russ. J. Phys. Chem. A 90, 1574 (2016).
I. K. Garkushin and A. V. Kolyado, Russ. J. Phys. Chem. A 91, 1146 (2017).
I. G. Yakovlev, I. K. Garkushin, and A. V. Kolyado, Russ. J. Phys. Chem. A 91, 1146 (2017).
I. K. Garkushin, E. V. Dorokhina, and A. V. Kolyado, Butler. Soobshch. 16 (3), 41 (2009).
A. G. Morachevskii, The Thermodynamics of Liquid–Vapor Equilibria (Khimiya, Leningrad, 1989) [in Russian].
Yu. V. Moshchenskii, Prib. Tekh. Eksp., No. 6, 143 (2003).
DSC Microcalorimeter, Laboratory Guidelines (Samar. Gos. Tekhn. Univ., Samara, 2004), p. 19 [in Russian].
S. V. Fedotov and Yu. V. Moshchenskii, Interface Software DSC Tool: User Guide (SamGTU, Samara, 2004), p. 23 [in Russian].
I. G. Yakovlev, I. K. Garkushin, and A. V. Kolyado, J. Thermal Anal. Calorim. 131, 455 (2018).
GOST (State Standard) No. 18995.2-73, Liquid chemical products. Refractive index method (2009).
V. M. Tatevskii, Physico-Chemical Properties of Individual Hydrocarbons (Gostoptekhizdat, Moscow, 1960) [in Russian].
I. K. Garkushin, A. V. Kolyado, and E. V. Dorokhina, Calculation and Study of Phase Equilibria in Binary Systems of Organic Substances (UrO RAN, Yekaterinburg, 2011) [in Russian].
I. K. Garkushin, A. V. Kolyado, and I. G. Yakovlev, in Proceedings of the International Conference on Theoretical and Applied Issues of Education and Science (Tambov, 2014), p. 186.
ACKNOWLEDGMENTS
The authors are grateful to S. I. Yakovleva for her assistance in preparing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakovlev, I.G., Garkushin, I.K. & Kolyado, A.V. Phase Diagrams of the Diphenyl Oxide–n-Hexadecane System. Russ. J. Phys. Chem. 94, 1556–1559 (2020). https://doi.org/10.1134/S0036024420080336
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420080336