Abstract
Main conclusion
Accumulation of specific metabolites, mainly γ-aminobutyric acid, polyamines, and proline, was essential to homeostasis regulation and differential salt tolerance in sorghum genotypes.
Abstract
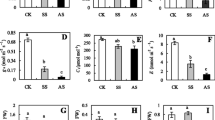
Salinity is severe abiotic stress that limits plant growth and development in arid and semi-arid regions. Survival to abiotic stresses depends on metabolic and sometimes even morphological adjustments. We measured the growth parameters, water relations, the content of ions (Na+, K+, Cl–), compatible solutes [some free amino acids (FAAs) including γ-aminobutyric acid (GABA) and proline and soluble carbohydrates) and polyamines (PAs), the activity of PAs metabolism enzymes, and metabolomic profile in plants after 14 days of salt stress treatment. These analyses were to evaluate the influence of metabolomic responses of sorghum genotypes exhibiting sensitivity (CSF18) or tolerance (CSF20) to salinity on plant growth. The salinity promoted growth reductions and induced increases in Na+ and Cl– content and decreases in K+ content. The water status and osmotic potential (Ψo) were reduced by salt stress, but to minimize damage, especially in the CSF20, the osmolytes and PAs contributed to the osmotic adjustment. The results showed that salinity induced an increase in putrescine (Put) in the sensitive genotype. However, it raised spermidine (Spd), spermine (Spm), and cadaverine (Cad) in the tolerant genotype. In addition, the regulation of polyamine oxidase can be related to Spm and GABA biosynthesis. Differential metabolic changes to salt tolerance include metabolites associated with tricarboxylic acid (TCA) cycle intermediates and the metabolisms of sugars, FAAs, and PAs.









Similar content being viewed by others
Abbreviations
- ADC:
-
Arginine decarboxylase
- Cad:
-
Cadaverine
- DAO:
-
Diamine oxidase
- PAO:
-
Polyamine oxidase
- DM:
-
Dry mass
- FAAs:
-
Free amino acids
- GABA:
-
γ-Aminobutyric acid
- PAs:
-
Polyamines
- Put:
-
Putrescine
- Spd:
-
Spermidine
- Spm:
-
Spermine
- Ψo :
-
Osmotic potential
References
Al Mahmud J, Hasanuzzaman M, Nahar K, Rahman A, Hossain MS, Fujita M (2017) Maleic acid assisted improvement of metal chelation and antioxidant metabolism confers chromium tolerance in Brassica juncea L. Ecotox Environ Safe 144:216–226
Al-Quraan NA, Sartawe FA, Qaryout MM (2013) Characterization of gamma-aminobutyric acid metabolism and oxidative damage in wheat (Triticum aestivum L.) seedlings under salt and osmotic stress. J Plant Physiol 170(11):1003–1009
Arif MA, Alseekh S, Harb J, Fernie A, Frank W (2018) Abscisic acid, cold and salt stimulate conserved metabolic regulation in the moss Physcomitrella patens. Plant Biol 20(6):1014–1022
Assaha D, Ueda A, Saneoka H, Al-Yahyai R, Yaish M (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Blum A (1989) Osmotic adjustment and growth of barley genotypes under drought stress. Crop Sci 29(1):230–233
Borrelli GM, Fragasso M, Nigro F, Platani C, Papa R, Beleggia R, Trono D (2018) Analysis of metabolic and mineral changes in response to salt stress in durum wheat (Triticum turgidum ssp. durum) genotypes, which differ in salinity tolerance. Plant Physiol Biochem 133:57–70
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65(5):1241–1257
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Cataldo DA, Haroonh M, Shrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic. Commun Soil Sci Plan 6(1):71–80
Čatský J (1960) Determination of water deficit in discs cut out from leaf blades. Biol Plant 2(1):76
Chebrolu KK, Fritschi FB, Ye S, Krishnan HB, Smith JR, Gillman JD (2016) Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 12:28
Cheng B, Li Z, Liang L, Cao Y, Zeng W, Zhang X, Ma X, Huang L, Nie G, Liu W, Peng Y (2018) The γ-aminobutyric acid (GABA) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int J Mol Sci 19(9):2520
Clark RB (1982) Nutrient solution growth of sorghum and corn in mineral nutrition studies. J Plant Nutr 5(8):1039–1057
Cuin TA, Shabala S (2007) Amino acids regulate salinity-induced potassium efflux in barley root epidermis. Planta 225(3):753–761
De Diego N, Sampedro MC, Barrio RJ, Saiz-Fernández I, Moncaleán P, Lacuesta M (2013) Solute accumulation and elastic modulus changes in six radiata pine breeds exposed to drought. Tree Physiol 33(1):69–80
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228
Duan J, Li J, Guo S, Kang Y (2008) Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J Plant Physiol 165(15):1620–1635
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Ebeed HT, Hassan NM, Aljarani AM (2017) Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol Biochem 118:438–448
Finiti I, Leyva MO, Vicedo B, Gómez-Pastor R, López-Cruz J, García-Agustín P, Real MD, González-Bosch C (2014) Hexanoic acid protects tomato plants against Botrytis cinerea by priming defence responses and reducing oxidative stress. Mol Plant Pathol 15(6):550–562
Freitas VS, Miranda RS, Costa JH, Farias de Oliveira D, Paula SO, Castro E, Freire RS, Prisco JT, Gomes-Filho E (2018) Ethylene triggers salt tolerance in maize genotypes by modulating polyamine catabolism enzymes associated with H2O2 production. Environ Exp Bot 145:75–86
Gaines TP, Parker MB, Gascho GJ (1984) Automated determination of chlorides in soil and plant tissue by sodium nitrate extraction. Agron J 76(3):371–374
Gong Q, Li P, Ma S, Indu Rupassara S, Bohnert HJ (2005) Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J 44(5):826–839
Hamouda I, Badri M, Mejri M, Cruz C, Siddique KHM, Hessini K (2016) Salt tolerance of Beta macrocarpa is associated with efficient osmotic adjustment and increased apoplastic water content. Plant Biol 18(3):369–375
Holmstedt B, Larsson L, Tham R (1961) Further studies on spectrophotometric method for the determination of amine oxidase activity. Biochim Biophys Acta 48:182–186
Hossain MS, Persicke M, ElSayed AI, Kalinowski J, Dietz KJ (2017) Metabolite profiling at the cellular and subcellular level reveals metabolites associated with salinity tolerance in sugar beet. J Exp Bot 68(21–22):5961–5976
Igamberdiev AU, Kleczkowski LA (2018) The glycerate and phosphorylated pathways of serine synthesis in plants: the branches of plant glycolysis linking carbon and nitrogen metabolism. Front Plant Sci 9:318
Isayenkov SV, Maathuis FJM (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80
Jacoby RP, Taylor NL, Millar AH (2011) The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci 16(11):614–623
Jiang D, Hao M, Fu J, Liu K, Yan X (2019) Potential bioethanol production from sweet sorghum on marginal land in China. J Clean Prod 220:225–234
Kitaoka S, Nakano Y (1969) Colorimetric determination of ω-amino acids. J Biochem 66(1):87–94
Kumar R (2009) Role of naturally occurring osmolytes in protein folding and stability. Arch Biochem Biophys 491(1–2):1–6
Kumar V, Khare T (2015) Individual and additive effects of Na+ and Cl− ions on rice under salinity stress. Arch Agron Soil Sci 61(3):381–395
Kumar V, Khare T, Shaikh S, Wani SH (2018) Compatible solutes and abiotic stress tolerance in plants. In: Ramakrishna A, Gill SS (eds) Metabolic adaptations in plants during abiotic stress. CRC Press, Boca Raton, pp 213–220
Kuznetsov V, Shorina M, Aronova E, Stetsenko L, Rakitin V, Shevyakova N (2007) NaCl- and ethylene-dependent cadaverine accumulation and its possible protective role in the adaptation of the common ice plant to salt stress. Plant Sci 172(2):363–370
Lacerda CF, Cambraia J, Cano MO, Ruiz HA (2001) Plant growth and solute accumulation and distribution in two sorghum genotypes, under NaCl stress. Rev Bras Fisiol Veg 13(3):270–284
Lacerda CF, Cambraia J, Cano MAO, Ruiz HA, Prisco JT (2003) Solute accumulation and distribution during shoot and leaf development in two sorghum genotypes under salt stress. Environ Exp Bot 49(2):107–120
Legocka J, Kluk A (2005) Effect of salt and osmotic stress on changes in polyamine content and arginine decarboxylase activity in Lupinus luteus seedlings. J Plant Physiol 162(6):662–668
Lei P, Xu Z, Liang J, Luo X, Zhang Y, Feng X, Xu H (2016) Poly(γ-glutamic acid) enhanced tolerance to salt stress by promoting proline accumulation in Brassica napus L. J Plant Growth Regul 78(2):233–241
Li S, Jin H, Zhang Q (2016) The effect of exogenous spermidine concentration on polyamine metabolism and salt tolerance in zoysiagrass (Zoysia japonica Steud) subjected to short-term salinity stress. Front Plant Sci 7:1221
Liang W, Ma X, Wan P, Liu L (2018) Plant salt-tolerance mechanism: a review. Biochem Biophys Res Commun 495(1):286–291
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1(1):387–396
Liu D, Ford KL, Roessner U, Natera S, Cassin AM, Patterson JH, Bacic A (2013) Rice suspension cultured cells are evaluated as a model system to study salt responsive networks in plants using a combined proteomic and metabolomic profiling approach. Proteomics 13(12–13):2046–2062
Liu T, Dobashi H, Kim DW, Sagor GHM, Niitsu M, Berberich T, Kusano T (2014) Arabidopsis mutant plants with diverse defects in polyamine metabolism show unequal sensitivity to exogenous cadaverine probably based on their spermine content. Physiol Mol Biol Plants 20(2):151–159
Mansour MMF, Ali EF (2017) Evaluation of proline functions in saline conditions. Phytochemistry 140:52–68
Marchetti CF, Ugena L, Humplík JF, Polak M, Podlešáková K, Fürst T, De Diego N, Spichal L (2019) A novel image-based screening method to study water deficit response and recovery of barley populations using canopy dynamics phenotyping and simple metabolite profiling. Front Plant Sci 10:1252
Meringer MV, Villasuso AL, Margutti MP, Usorach J, Pasquaré SJ, Giusto NM, Machado EE, Racagni GE (2016) Saline and osmotic stresses stimulate PLD/diacylglycerol kinase activities and increase the level of phosphatidic acid and proline in barley roots. Environ Exp Bot 128:69–78
Misra N, Saxena P (2009) Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci 177(3):181–189
Misra N, Gupta K, Dwivedi UN (2006) Changes in free amino acids and stress protein synthesis in two genotypes of green gram under salt stress. J Plant Sci 1(1):56–66
Mona SA, Hashem A, Abd_Allah EF, Alqarawi AA, Soliman DWK, Wirth S, Egamberdieva D (2017) Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J Integr Agric 16(8):1751–1757
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Safe 60(3):324–349
Parida AK, Panda A, Rangani J (2018) Metabolomics-guided elucidation of abiotic stress tolerance mechanisms in plants. In: Ahmad P, Ahanger MA, Singh VP, et al. (eds) Plant metabolites and regulation under environmental stress. Academic Press, Cambridge, pp 89–131
Pathak MR, Silva JAT, Wani SH (2014) Polyamines in response to abiotic stress tolerance through transgenic approaches. GM Crops Food 5(2):87–96
Pérez-López U, Robredo A, Lacuesta M, Sgherri C, Mena-Petite A, Navari-Izzo F, Muñoz-Rueda A (2010) Lipoic acid and redox status in barley plants subjected to salinity and elevated CO2. Physiol Plant 139(3):256–268
Podlešáková K, Ugena L, Spíchal L, Doležal K, De Diego N (2019) Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol 48:53–65
Puyang X, An M, Xu L, Han L, Zhang X (2016) Protective effect of exogenous spermidine on ion and polyamine metabolism in Kentucky bluegrass under salinity stress. Hortic Environ Biotechnol 57(1):11–19
Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13(1):11–29
Ronda V, Aruna C, Visarada K, Bhat B (2018) Sorghum for animal feed. In: Aruna C, Visarada K, Bhat B, Tonapi V (eds) Breeding sorghum for diverse end uses. Woodhead Publishing, Cambridge, pp 229–238
Sah SK, Kaur G, Wani SH (2016) Metabolic engineering of compatible solute trehalose for abiotic stress tolerance in plants. In: Iqbal N, Nazar R, Khan AN (eds) Osmolytes and plants acclimation to changing environment: emerging omics technologies. Springer, New Delhi, pp 83–96
Sami F, Yusuf M, Faizan M, Faraz A, Hayat S (2016) Role of sugars under abiotic stress. Plant Physiol Biochem 109:54–61
Scalschi L, Vicedo B, Camañes G, Fernandez-Crespo E, Lapeña L, González-Bosch C, García-Agustín P (2013) Hexanoic acid is a resistance inducer that protects tomato plants against Pseudomonas syringae by priming the jasmonic acid and salicylic acid pathways. Mol Plant Pathol 14(4):342–355
Seifikalhor M, Aliniaeifard S, Hassani B, Niknam V, Lastochkina O (2019) Diverse role of γ-aminobutyric acid in dynamic plant cell responses. Plant Cell Rep 38(8):847–867
Shabala S (2017) Signalling by potassium: another second messenger to add to the list? J Exp Bot 68(15):4003–4007
Shahid SA, Zaman M, Heng L (2018) Soil salinity: historical perspectives and a world overview of the problem. Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques. Springer, Cham, pp 43–53
Sudhakar C, Veeranagamallaiah G, Nareshkumar A, Sudhakarbabu O, Sivakumar M, Pandurangaiah M, Kiranmai K, Lokesh U (2015) Polyamine metabolism influences antioxidant defense mechanism in foxtail millet (Setaria italica L.) cultivars with different salinity tolerance. Plant Cell Rep 34(1):141–156
Suprasanna P, Nikalje GC, Rai AN (2016) Osmolyte accumulation and implications in plant abiotic stress tolerance. In: Iqbal N, Nazar R, Khan NA (eds) Osmolytes and plants acclimation to changing environment: emerging omics technologies. Springer, New Delhi, pp 1–12
Wang W, Paschalidis K, Feng J, Song J, Liu J (2019) Polyamine catabolism in plants: a universal process with diverse functions. Front Plant Sci 10:561
Wang J, Jiang X, Zhao C, Fang Z, Jiao P (2020) Transcriptomic and metabolomic analysis reveals the role of CoA in the salt tolerance of Zygophyllum spp. BMC Plant Biol 20(1):1–14
Wu J, Shu S, Li C, Sun J, Guo S (2018) Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol Biochem 128:152–162
Yang Y, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol 217(2):523–539
Yemm EW, Cocking EC (1955) The determination of aminoacids with ninhydrin. Analyst 80(948):209–213
Yin L, Wang S, Tanaka K, Fujihara S, Itai A, Den X, Zhang S (2016) Silicon-mediated changes in polyamines participate in silicon-induced salt tolerance in Sorghum bicolor L. plant. Plant Cell Environ 39(2):245–258
Acknowledgements
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Instituto Nacional de Ciência e Tecnologia em Salinidade (INCTsal) by financial support. DFO thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for fellowship during his Doctoral studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Oliveira, D.F., Lopes, L.d. & Gomes-Filho, E. Metabolic changes associated with differential salt tolerance in sorghum genotypes. Planta 252, 34 (2020). https://doi.org/10.1007/s00425-020-03437-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03437-8