Abstract
Neuronostatin (NST) is an endogenous peptide hormone, it has the ability to improve oligomeric Aβ (oAβ)-induced cognitive impairments and increase blood glucose levels in mice. However, the relationship between NST and oAβ regarding brain glucose metabolism has not yet been established. The present study defined the contributions of NST and oAβ in the brain glucose metabolism in mice. It was found that i.c.v. co-administration of NST (3 nmol/mouse) and oAβ (1 nmol/mouse) decreased the mRNA expressions of glucose-6-phosphate dehydrogenase and phosphofructokinase. The treatments were observed to reduce ATP production and the enzyme activities of glucose-6-phosphate dehydrogenase and hexokinase in both the cortex and hippocampus. Simultaneously, co-injection of NST and oAβ inhibited the mRNA and protein expression of glucose transporters GLUT3 and GLUT1 in the cortex and hippocampus. NST promoted the oAβ-induced decreased the cortical NeuN staining, while oAβ increased the levels of NST in both the cortex and hippocampus. I.c.v. co-administration of NST and oAβ led to increase the levels of GPR107 expression and the phosphorylation of PKA, Akt, PERK and eIF-2α in the cortex. These findings suggest that NST promoted oAβ-induced dysfunctional glucose metabolism through the GPR107/PKA/Akt signaling pathway and PERK/eIF2α axis in the brain, which thus contributes to metabolic dysfunction and Alzheimer’s disease (AD) pathophysiology.




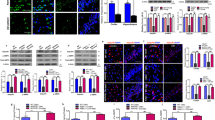
(a) and hippocampus (b), which were measured using western blot for the immunoblots of GLUT1 and GLUT3, the immunoreactivity of GLUT1 was normalized to β-actin, and GLUT3 to GAPDH. The data are represented as the mean ± SEM from four individual mice in each group. *Indicates P < 0.05 and **Indicates P < 0.01, which are compared to the controls by the Tukey’s test; #Indicates P < 0.05 between oAβ and NST + oAβ by Student’s t test


Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- oAβ:
-
Aβ42 oligomers
- ECL:
-
Chemiluminescence
- eIF2α:
-
Eukaryotic initiation factor 2α
- FDG:
-
Fluorodeoxyglucose
- GSK3β:
-
Glucogen synthase kinase 3
- G6PD:
-
Glucose-6-phosphate dehydrogenase
- GLUT:
-
Glucose transporter
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GPR107:
-
G-protein-coupled receptor 107
- HK:
-
Hexokinase
- I.c.v.:
-
Intracerebroventricular
- NST:
-
Neuronostatin
- PPP:
-
Pentose phosphate pathway
- PFK:
-
Phosphofructokinase
- PET:
-
Positron emission tomography
- PKA:
-
Protein kinase A
- Akt:
-
Protein kinase B
- PERK:
-
PKR-like ER kinase
- RT-qPCR:
-
Quantitative real-time PCR
- ROS:
-
Reactive oxygen species
- SST:
-
Somatostatin
- TBP:
-
TATA binding protein
- TCA:
-
Tricarboxylic acid
- UPR:
-
Unfolded protein response
- HFIP:
-
1,1,1,3,3,3-Hexafluoro-2-propanol
References
Luque RM, Kineman RD (2018) Neuronostatin exerts actions on pituitary that are unique from its sibling peptide somatostatin. J Endocrinol 237:217–227. https://doi.org/10.1530/JOE-18-0135
Yang SB, Yang AM, Su SF et al (2012) Neuronostatin induces hyperalgesia in formalin test in mice. Neurosci Lett 506:126–130. https://doi.org/10.1016/j.neulet.2011.10.064
Yosten GL, Redlinger LJ, Samson WK (2012) Evidence for an interaction of neuronostatin with the orphan G protein-coupled receptor, GPR107. Am J Physiol Regul Integr Comp Physiol 303:941–949. https://doi.org/10.1152/ajpregu.00336.2012
Samson WK, Zhang JV, Avsian-Kretchmer O et al (2008) Neuronostatin encoded by the somatostatin gene regulates neuronal, cardiovascular and metabolic functions. J Biol Chem 283:31949–31959. https://doi.org/10.1074/jbc.M804784200
Dun SL, Brailoiu GC, Tica AA et al (2010) Neuronostatin is co-expressed with somatostatin and mobilizes calcium in cultured rat hypothalamic neurons. Neuroscience 166:455–463
Carlini VP, Ghersi M, Gabach L et al (2011) Hippocampal effects of neuronostatin on memory, anxiety-like behavior and food intake in rats. Neuroscience 197:145–152
Yang SB, Shao TJ, Yu P et al (2019) Neuronostatin promotes soluble Aβ1-42 oligomers–induced spatial learning and memory impairments in mice. Behav Brain Res 364:62–74. https://doi.org/10.1016/j.bbr.2019.01.047
Elrick MM, Samson WK, Corbett JA et al (2016) Neuronostatin acts via GPR107 to increase cAMP-independent PKA phosphorylation and proglucagon mRNA accumulation in pancreatic α-cells. Am J Physiol Regul Integr Comp Physiol 310:143–155. https://doi.org/10.1152/ajpregu.00369.2014
Salvatori AS, Elrick MM, Samson WK et al (2014) Neuronostatin inhibits glucose-stimulated insulin secretion via direct action on the pancreatic α-cell. Am J Physiol Endocrinol Metab 306:1257–1263. https://doi.org/10.1152/ajpendo.00599.2013
Nussbaum RL, Ellis CE (2003) Alzheimer's disease and Parkinson's disease. N Engl J Med 348:1356–1364
Neth BJ, Craft S (2017) Insulin resistance and Alzheimer's disease: bioenergetic linkages. Front Aging Neurosci 9:345. https://doi.org/10.3389/fnagi.2017.00345
De Felice C, Della RF et al (2014) Oxidative brain damage in Mecp2-mutant murine models of Rett syndrome. Neurobiol Dis 68:66–77. https://doi.org/10.1016/j.nbd.2014.04.006
Langbaum JB, Chen K, Lee W et al (2009) Categorical and correlational analyses of baseline fluorodeoxyglucose positron emission tomography images from the Alzheimer's Disease Neuroimaging Initiative (ADNI). Neuroimage 45:1107–1116. https://doi.org/10.1016/j.neuroimage.2008.12.072
Arvanitakis Z, Wilson R, Bienias JL et al (2004) Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol 61:661–666
Lu Y, Ren J, Cui S et al (2016) Cerebral glucose metabolism assessment in rat models of Alzheimer's disease: an 18F-FDG-PET study. Am J Alzheimers Dis Other Demen 31:333–340. https://doi.org/10.1177/1533317515617725
Waldron AM, Wintmolders C, Bottelbergs A et al (2015) In vivo molecular neuroimaging of glucose utilization and its association with fibrillar amyloid-β load in aged APPPS1-21 mice. Alzheimers Res Ther 7:76. https://doi.org/10.1186/s13195-015-0158-6
Wang D, Li X, Gao K et al (2013) Cardiotrophin-1 (CTF1) ameliorates glucose-uptake defects and improves memory and learning deficits in a transgenic mouse model of Alzheimer's disease. Pharmacol Biochem Behav 107:48–57. https://doi.org/10.1016/j.pbb.2013.03.003
Niccoli T, Cabecinha M, Tillmann A et al (2016) Increased glucose transport into neurons rescues abeta toxicity in Drosophila. Curr Biol 26:2291–2300
Liu W, Zhuo P, Li L et al (2017) Activation of brain glucose metabolism ameliorating cognitive impairment in APP/PS1 transgenic mice by electroacupuncture. Free Radical Biol Med 112:174–190
Mark RJ, Pang Z, Geddes JW et al (1997) Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci 17:1046–1054
Hayes JD, McLellan LI (1999) Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 31:273–300
Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, Anagnostou V, Rimm D (2010) Antibody validation. Biotechniques 48:197–209. https://doi.org/10.2144/000113382
Han RW, Zhang RS, Xu HJ et al (2013) Neuropeptide S enhances memory and mitigates memory impairment induced by MK801, scopolamine or Aβ1-42 in mice novel object and object location recognition tasks. Neuropharmacology 70:261–267. https://doi.org/10.1016/j.neuropharm.2013.02.002
Zurashvili T, Cordon-Barris L, Ruiz-Babot G et al (2013) Interaction of PDK1 with phosphoinositides is essential for neuronal differentiation but dispensable for neuronal survival. Mol Cell Biol 33:1027–1040
Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates. Academic Press
Hewitt SM, Baskin DG, Frevert CW et al (2014) Controls for immunohistochemistry: the histochemical society's standards of practice for validation of immunohistochemical assays. J Histochem Cytochem 62(10):693–697. https://doi.org/10.1369/0022155414545224
Kleinridders A, Ferris HA, Reyzer ML et al (2018) Regional differences in brain glucose metabolism determined by imaging mass spectrometry. Mol Metab 12:113–121. https://doi.org/10.1016/j.molmet.2018.03.013
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Castellano CA, Nugent S, Paquet N et al (2015) Lower brain 18F-fluorodeoxyglucose uptake but normal 11C-acetoacetate metabolism in mild Alzheimer's disease dementia. J Alzheimers Dis 43:1343–1353. https://doi.org/10.3233/JAD-141074
Vannucci SJ, Maher F, Simpson IA (1997) Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia 21:2–21
Belanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738
Dias C, Lourenco CF, Ferreiro E et al (2016) Age-dependent changes in the glutamate-nitric oxide pathway in the hippocampus of the triple transgenic model of Alzheimer's disease: implications for neurometabolic regulation. Neurobiol Aging 46:84–95. https://doi.org/10.1016/j.neurobiolaging.2016.06.012.34
Williams CR, Gooch JL (2014) Calcineurin Aβ regulates NADPH oxidase (Nox) expression and activity via nuclear factor of activated T cells (NFAT) in response to high glucose. J Biol Chem 289:4896–4905. https://doi.org/10.1074/jbc.M113.514869
Yang SB, Pascual-Guiral S, Ponce R et al (2017) Reducing the levels of Akt activation by PDK1 knock-in mutation protects neuronal cultures against synthetic amyloid-beta peptides. Front Aging Neurosci 9:435
Mercado G, Valdés P, Hetz C (2013) An ERcentric view of Parkinson's disease. Trends Mol Med 19:165–175. https://doi.org/10.1016/j.molmed.2012.12.005
Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15:233–249
Balsa E, Soustek MS, Thomas A et al (2019) ER and nutrient stress promote assembly of respiratory chain supercomplexes through the PERK-eIF2a axis. Mol Cell 74:877–890. https://doi.org/10.1016/j.molcel.2019.03.031
Ma T, Trinh MA, Wexler AJ et al (2013) Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nat Neurosci 16:1299–1305. https://doi.org/10.1038/nn.3486
Acknowledgements
This work was funded by Natural Science Foundation of Gansu Province (Grant No.18JR3RA101); Youth Science and Technology Talents Lifting Project Foundation of Gansu Province, and Doctoral Launching Foundation of Northwest Normal University.
Author information
Authors and Affiliations
Contributions
The conception and design of the work were prepared by YS and YP. The experiment was performed by YS, ZF, YY, MM, and ZS, while the analysis and interpretation of data were done by YS and YP. Drafting of the manuscript was done by YS and YP.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this study.
Ethical Approval
All experiments were conducted in compliance with the ARRIVE guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, S., Zhou, F., Ma, M. et al. Neuronostatin Promotion Soluble Aβ1-42 Oligomers: Induced Dysfunctional Brain Glucose Metabolism in Mice. Neurochem Res 45, 2474–2486 (2020). https://doi.org/10.1007/s11064-020-03106-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03106-y