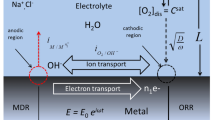
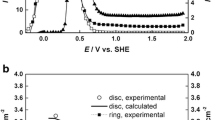
Abstract
It is known that the kinetics of redox reactions occurring on the surfaces of passive metals depend upon the properties of the passive film, ostensibly due to quantum mechanical tunnelling (QMT) of electrons and holes between the metal and the redox couple at the barrier layer/solution (bl/s) interface. In this paper, the tunnelling probability is used to inter-convert the exchange current densities for the redox reactions occurring at the bl/s interface and on the hypothetical bare metal surface. We review our previous work on combining QMT theory with the point defect model (PDM), which provides an analytical expression for the bl thickness as a function of voltage. By combining QMT theory and the PDM, we derive a modified form of the generalized Butler-Volmer equation that requires as input only the kinetic parameters for the redox reaction on the hypothetical bare surface and parameters contained in the PDM. The application of the theory is illustrated with reference to the corrosion of carbon steel in concrete pore solution, to calculating the corrosion potential of, and crack growth rate in, sensitized type 304 SS in boiling water reactor (BWR) coolant circuits, and the use of hydrogen oxidation on platinum to determine the thickness of the bl as a function of voltage and temperature. This illustrates a new, powerful technique for probing the formation of passive films on metal surfaces.










Similar content being viewed by others
References
Bockris JOM, Khan SUM Quantum Electrochemistry, Springer Nature, Basel
Erdey-Grüz T, Volmer M (1930) Zur Theorie der Wasserstoff Überspannung. Z Physik Chem A150:203
Butler JAV (1932) The mechanism of overvoltage and its relation to the combination of hydrogen atoms at metal electrodes. Trans Faraday Soc 27(379–382)
Vetter KJ, Schultze JW (1973) Charge carrier tunneling across the passive film on platinum. Ber Bunsenges Phys Chem 77:945–953
Schultze JW, Vetter KJ (1973) The influence of the tunnel probability on the anodic oxygen evolution and other redox reactions at oxide covered platinum electrodes. Electrochem Acta 18(11):889–896
Schuldiner SJ (1968) Oxidation of hydrogen on a passive platinum electrode. J Electrochem Soc 115(4):362–365
Moffat TP, Yang H, Fan RF, Bard AJ (1992) Electron-transfer reactions on passive chromium. J Electrochem Soc 139(11):3158–3167
Hamann CH (1971) Die Oxidation von Kohlenmonoxid an Platin im sauren Elektrolyten. Ber Bunsenges Phys Chem 75(6):542–547
Dickinson T, Greef R, Wynne-Jones L (1969) The kinetics of the chlorine electrode reaction at a platinum electrode. Electrochem Acta 14(6):467–489
Gurney RW (1931) The quantum mechanics of electrolysis. Proc R Soc A 134:137–154
Gerischer H (1960) Z. Über den Ablauf von Redoxreaktionen an Metallen und an Halbleitern. II. Metall-Elektroden. Phys. Chem. N.F. 26: 223–247, 325–338
Bao J, Macdonald DD (2007) Oxidation of hydrogen on oxidized platinum. J Electroanal Chem 600(1):205–216
Macdonald DD (1992) The point defect model for the passive state. J Electrochem Soc 139(12):3434–3449
Macdonald DD (2011) The history of the point defect model for the passive state: a brief review of film growth aspects. Electrochim Acta 56(4):1761–1772
Macdonald DD (2012) Some personal adventures in passivity—a review of the point defect model for film growth. Elektrokhimiya 48(3):259–284
Macdonald DD, Engelhardt DD (2010) The point defect model for bi-layer passive films. ECS Trans 28:24123–24144
Macdonald DD (1999) Passivity: the key to our metals-based civilization. Pure Appl Chem 71(6):951–986
Macdonald DD (2012) The passive state in our reactive metals-based civilization. Arab J Sci Eng 37(5):1143–1185
Macdonald DD, Sun A (2006) An electrochemical impedance spectroscopic study of the passive state on Alloy-22, Electrochim. Acta 51:1767–1779
Sharifi-Asl S, Taylor M, Lu Z, Engelhardt GR, Kursten B, Macdonald DD (2013) Modeling of the electrochemical impedance spectroscopic behavior of passive iron using a genetic algorithm approach. Electrochim Acta 102:161–173
Lin LF, Chao CY, Macdonald DD (1981) A point-defect model for anodic passive films: II. Chemical Breakdown and Pit Initiation. J Electrochem Soc 128:1194–1198
Selman JR, Tobias CW (1978) Mass-transfer measurements by the limiting-current technique. Adv Chem Eng Sci 10:211–318
Angst UM, Eisner B, Larsen CK, Vennesland O (2011) Chloride induced reinforcement corrosion: electrochemical monitoring of initiation stage and chloride threshold values. Corros Sci 53(4):1451–1464
Sagüés AA, Pech-Canul MA, Al-Mansur S (2003) Corrosion macrocell behavior of reinforcing steel in partially submerged concrete columns. Corros Sci 45(1):7–32
Bao JE, Macdonald DD (2013) Growth kinetics of the anodic oxide film on platinum under potentiodynamic polarization conditions. Z Physik Chem 227(5):541–559
Lu P, Sharifi-Asl S, Kursten B, Macdonald DD (2015) The irreversibility of the passive state of carbon steel in the alkaline concrete pore solution under simulated anoxic conditions. J Electrochem Soc 162:C572–C581
Qiu J, Li Y, Xu Y, Wu A, Macdonald DD (2020) Effect of temperature on corrosion of carbon steel in simulated concrete pore solution under anoxic conditions. Corros Sci Accepted, 108886
Sze S (1985) Semiconductor devices: physics and technology, 2nd edn. Wiley, New York
Macdonald DD, Lu PC, Urquidi-Macdonald M, Yeh TK (1996) Theoretical estimation of crack growth rates in type 304 stainless steel in boiling-water reactor coolant environments. Corrosion 52(10):768–785
Lee SK, Lv P, Macdonald DD (2013) Customization of the CEFM for predicting stress corrosion cracking in lightly sensitized Al–Mg alloys in marine applications. J Solid State Electrochem 17(8):2319–2332
Maeng WY, Macdonald DD (2008) The effect of acetic acid on the stress corrosion cracking of 3.5NiCrMoV turbine steels in high temperature water. Corros Sci 50(8):2239–2250
Lee SK, Macdonald DD (2013) Stress Corrosion Cracking of Alloy 22. In: 224th ECS Meeting. The Electrochemical Society, San Francisco, p 1781
Shi J, Wang J, Macdonald DD (2014) Prediction of crack growth rate in type 304 stainless steel using artificial neural networks and the coupled environment fracture model. Corros Sci 89:69–80
Macdonald DD (1992) Viability of hydrogen water chemistry for protecting in-vessel components of boiling water reactors. Corrosion 48(3):194–205
Sutanto F, Macdonald DD (2011) On the role of quantum mechanics in corrosion processes. Proc. Austral. Corros. Assoc, Auckland
Macdonald DD, Urquidi-Macdonald M (1991) Proc. Fifth Int. Symp. Envir. Degr. Mat. Nucl. Power Systems-Water Reactors, pp. 345-349. Monterey, CA, ANS/NACE. Houston, TX, National Association of Corrosion Engineers, International
Macdonald DD, Urquidi-Macdonald M (1991) A coupled environment model for stress corrosion cracking in sensitized type 304 stainless steel in LWR environments. Corros Sci 32(1):51–81
Lu PC, Macdonald DD and Urquidi-Macdonald M (1994) Corrosion/94, NACE Annual Conference and Corrosion Show. Paper #246. pp. 1–22. NACE International, Houston, TX
Manahan MP, Macdonald DD, Peterson AJ Jr (1995) Determination of the fate of the current in the stress corrosion cracking of sensitized type 304SS in high temperature aqueous systems. Corros Sci 37(1):189–208
Balachov I, Macdonald DD (1998) Enhancing the operation of boiling water reactors by deterministic simulation. Proc. Water Chemistry ‘98, 1998 JAIF Int. Conf. Water Chem. Nucl. Power plants, Kashiwazaki, Japan, Oct. 13-16
Balachov I, Henzel N, Kilian R, Macdonald DD, Stellwag B (1998) Prediction of materials damage history from stress corrosion cracking in boiling water reactors. In: Proc. ASME/JSME Joint Press, vol 376. Vess. Piping Conf, San Diego, pp 101–109
Macdonald DD, Balachov I, Engelhardt G (1999) Deterministic prediction of localized corrosion damage in power plant coolant circuits. Power Plant Chem 1(1):9–16
Zhou XY, Balachov I, Macdonald DD (1998) The effect of dielectric coatings on IGSCC in sensitized type 304 SS in high temperature dilute sodium sulfate solution. Corros Sci 40(8):1349–1362
Acknowledgements
The authors gratefully acknowledge the support of this work by the University of California at Berkeley and by ONDRAF-NIRAS of Belgium.
Availability of data and materials
All sources of data and material are cited in the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Code availability
All codes used in data treatment are identified in the citation and are available from the original sources.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Macdonald, D.D., Qiu, J. Re-defining the kinetics of redox reactions on passive metal surfaces. J Solid State Electrochem 24, 2663–2677 (2020). https://doi.org/10.1007/s10008-020-04791-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04791-z