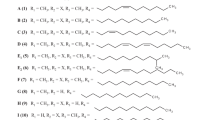
Abstract
Peptidoglycan, an essential component of the bacterial cell wall plays a critical role in protecting bacteria against osmotic lysis. The ATP-dependent MurC-F ligases are crucial for the early stages of peptidoglycan biosynthesis. MurE ligase is third in the series and catalyzes the addition of l-Lysine (l-Lys) in Gram-positive bacteria or meso-diaminopimelic acid (meso-A2pm) in most Gram-negative bacteria to form UDP-N-acetylmuramoyl-l-Ala-d-Glu-l-Lys/A2pm. The high substrate specific for l-Lys or meso-A2pm makes this enzyme an attractive target for the development of antibacterial agents. Several MurE inhibitors have been reported including phosphinates, peptidosulfonamides, napthylfuran-2-ones, benzene-1,3-dicarboxylic acids, phosphorylatedhydroxyethylamines, natural compounds, 5-benzylidenethiazolidin-4-ones, N-alkyl-2-alkynyl-4(1H)-quinolones, rhodanine substituted d-glutamic acids, 2,5-dimethyl pyrroles, 2,5-disubstitued furans, tetrahydroisoquinolines etc. In the present review we present an update status and structural information of MurE enzyme inhibitors which may be utilized for the design of potent inhibitors against this enzyme.













Similar content being viewed by others
Abbreviations
- ATP:
-
Adenosine triphosphate
- d-Glu:
-
d-Glutamic acid
- IC50 :
-
Half maximal inhibitory concentration
- l-Ala:
-
l-Alanine
- meso-A2pm:
-
meso-Diaminopimelic acid
- MIC:
-
Minimum inhibitory concentration
- MurE:
-
UDP-N-acetylmuramoyl-l-Ala:d-Glu ligase
- UDP:
-
Uridine-5′-diphosphate
- UDP-MurNAc:
-
UDP-N-acetylmuramic acid
References
Jacobs, M.R.: Worldwide trends in antimicrobial resistance among common respiratory tract pathogens in children. Pediatr. Infect. Dis. J. 22, 109–119 (2003)
Silver, L.L.: Novel inhibitors of bacterial cell wall synthesis. Curr. Opin. Microbiol. 6, 431–438 (2003)
Gordon, E., Flouret, B.: Crystal structure of UDP-N-acetylmuramoyl-l-alanyl-d-glutamate: meso-diaminopimelate ligase from Escherichia coli. J. Biol. Chem. 276, 10999–11006 (2001)
Boniface, A., Bouhss, A.: The MurE synthetase from Thermotoga maritima is endowed with an unusual d-lysine adding activity. J. Biol. Chem. 281, 15680–15686 (2006)
Triolo, T.A., Chabin, R.M., Pompliano, D.L.: Cloning, expression and characterization of the Streptococcus pyogenes murE gene encoding a UDP-MurNAc-l-alanyl-d-glutamate: l-lysine ligase. Enzym. Microb. Technol. 35, 300–308 (2004)
Mengin-Lecreulx, D., Falla, T., Blanot, D., van Heijenoort, J., Adams, D.J., Chopra, I.: Expression of the Staphylococcus aureus UDP-N-acetylmuramoyl-l-alanyld-glutamate:l-lysine ligase in Escherichia coli and effects on peptidoglycan biosynthesis and cell growth. J. Bacteriol. 181, 5909–5914 (1999)
Glauner, B., Holtje, J.V., Schwarz, U.: The composition of the murein of Escherichia coli. J. Boil. Chem. 263, 10088–10095 (1988)
Ruane, K.M., Lloyd, A.J.: Specificity determinants for lysine incorporation in Staphylococcus aureus peptidoglycan as revealed by the structure of a MurE enzyme ternary complex. J. Biol. Chem. 288, 33439–33448 (2013)
El-Zoeiby, A., Sanschagrin, F., Levesque, R.C.: Structure and function of the Mur enzymes: development of novel inhibitors. Mol. Microbiol. 47, 1–2 (2003)
Smith, C.A.: Structure, function and dynamics in the mur family of bacterial cell wall ligases. J. Mol. Biol. 362, 640–655 (2006)
Sheng, Y., Sun, X., Shen, Y., Bognar, A.L., Baker, E.N., Smith, C.A.: Structural and functional similarities in the ADP-forming amide bond ligase superfamily: implications for a substrate-induced conformational change in folylpolyglutamate synthetase. J. Mol. Biol. 302, 425–438 (2000)
Dementin, S., Bouhss, A., Auger, G., Parquet, C., Mengin-Lecreulx, D., Dideberg, O., van-Heijenoort, J., Blanot, D.: Evidence of a functional requirement for a carbamoylated lysine residue in MurD, MurE and MurF synthetases as established by chemical rescue experiment. Eur. J. Biochem. 268, 5800–5807 (2001)
Bouhss, A., Dementin, S., van Heijenoort, J., Parquet, C., Blanot, D.: Crystal structure of UDP-N-acetylmuramoyl-l-alanyl-d-glutamate: meso-diaminopimelate ligase from Escherichia coli. FEBS Lett. 453, 15–19 (1999)
Barreteau, H., Kovac, A., Boniface, A., Sova, M., Gobec, S., Blanot, D.: Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32, 168–207 (2008)
Van Falk, P.J., Ervin, K.M., Volk, K.S., Ho, H.T.: Biochemical evidence for the formation of a covalent acyl-phosphate linkage between UDP-N-acetylmuramate and ATP in the Escherichia coli UDP-N-acetylmuramate:l-alanine ligase-catalyzed reaction. Biochemistry 35, 1417–1422 (1996)
Perdih, A., Kotnik, M., Hodoscek, M., Solmajer, T.: Targeted molecular dynamics simulation studies of binding and conformational changes in E. coli MurD. Proteins 68, 243–254 (2007)
Bouhss, A., Dementin, S., van-Heijenoort, J., Parquet, C., Blanot, D.: MurC and MurD synthetases of peptidoglycan biosynthesis: borohydride trapping of acyl-phosphate intermediates. Methods Enzymol. 354, 189–196 (2002)
Williams, R.M., Fegley, G.J., Gallegos, R., Schaefer, F., Pruess, D.L.: Asymmetric syntheses of (2S,3S,6S), (2S,3S,6R)-, and (2R,3R,6S)-2,3-methano-2,6-diaminopimelic. Acids studies directed to the design of novel substrate-based inhibitors of l, l-diaminopimelate epimerase. Tetrahedron 52, 1149–1164 (1996)
Auger, G., van Heijenoort, J., Vederas, J.C., Blanot, D.: Effect of analogues of diaminopimelic acid on the meso-diaminopimelate-adding enzyme from Escherichia coli. FEBS Lett. 391, 171–174 (1996)
Longenecker, K.L., Stamper, G.F., Hajduk, P.J., Fry, E.H., Jakob, C.G., Harlan, J.E., Edalji, R., Bartley, D.M., Walter, K.A., Solomon, L.R., Holzman, T.F.: Structure of MurF from Streptococcus pneumoniae co-crystallized with a small molecule inhibitor exhibits interdomain closure. Protein Sci. 14, 3039–3047 (2005)
Tanner, M.E., Vaganay, S., van-Heijenoort, J., Blanot, D.: Phosphinate inhibitors of the d-glutamic acid-adding enzyme of peptidoglycan biosynthesis. J. Org. 61, 1756–1760 (1996)
Strancar, K., Blanot, D., Gobec, S.: Design, synthesis and structure–activity relationships of new phosphinate inhibitors of MurD. Bioorg. Med. Chem. Lett. 16, 343–348 (2006)
Hrast, M., Sosič, I., Šink, R., Gobec, S.: Inhibitors of the peptidoglycan biosynthesis enzymes MurA-F. Bioorg. Med. 55, 2–15 (2014)
Zeng, B., Wong, K.K., Pompliano, D.L., Reddy, S., Tanner, M.E.: A phosphinate inhibitor of the meso-diaminopimelic acid-adding enzyme (MurE) of peptidoglycan biosynthesis. J. Org. Chem. 63, 10081–10086 (1998)
Gegnas, L.D., Waddell, S.T., Chabin, R.M., Reddy, S., Wong, K.K.: Inhibitors of the bacterial cell wall biosynthesis enzyme Mur D. Bioorg. Med. Chem. Lett. 8, 1643–1648 (1998)
Humljan, J., Kotnik, M., Boniface, A., Šolmajer, T., Urleb, U., Blanot, D., Gobec, S.: A new approach towards peptidosulfonamides: synthesis of potential inhibitors of bacterial peptidoglycan biosynthesis enzymes MurD and MurE. Tetrahedron 62, 10980–10988 (2006)
Mansour, T.S., Caufield, C.E., Rasmussen, B., Chopra, R., Krishnamurthy, G., Morris, K.M., Svenson, K., Bard, J., Smeltzer, C., Naughton, S., Antane, S.: Naphthyltetronic acids as multi-target inhibitors of bacterial peptidoglycan biosynthesis. Chem. Med. Chem. 2, 1414–1417 (2007)
Perdih, A., Kovac, A., Wolber, G., Blanot, D., Gobec, S., Solmajer, T.: Discovery of novel benzene 1, 3-dicarboxylic acid inhibitors of bacterial MurD and MurE ligases by structure-based virtual screening approach. Bioorg. Med. Chem. Lett. 19, 2668–2673 (2009)
Sova, M., Kovac, A., Turk, S., Hrast, M., Blanot, D., Gobec, S.: Phosphorylated hydroxyethylamines as novel inhibitors of the bacterial cell wall biosynthesis enzymes MurC to MurF. Bioorg. Chem. 37, 217–222 (2009)
Guzman, J.D., Gupta, A., Evangelopoulos, D., Basavannacharya, C., Pabon, L.C., Plazas, E.A., Munoz, D.R., Delgado, W.A., Cuca, L.E., Ribon, W., Gibbons, S.: Anti-tubercular screening of natural products from Colombian plants: 3-methoxynordomesticine, an inhibitor of MurE ligase of Mycobacterium tuberculosis. J. Antimicrob. Chemother. 65, 2101–2107 (2010)
Tomasic, T., Zidar, N., Kovac, A., Turk, S., Simcic, M., Blanot, D., Muller-Premru, M., Filipic, M., Grdadolnik, S.G., Zega, A., Anderluh, M.: 5-Benzylidenethiazolidin-4-ones as multitarget inhibitors of bacterial Mur ligases. Chem. Med. Chem. 5, 286–295 (2010)
Guzman, J.D., Wube, A., Evangelopoulos, D., Gupta, A., Hüfner, A., Basavannacharya, C., Rahman, M.M., Thomaschitz, C., Bauer, R., McHugh, T.D., Nobeli, I.: Interaction of N-methyl-2-alkenyl-4-quinolones with ATP-dependent MurE ligase of Mycobacterium tuberculosis: antibacterial activity, molecular docking and inhibition kinetics. J. Antimicrob. Chemother. 66, 1766–1772 (2011)
Wube, A., Guzman, J.D., Hufner, A., Hochfellner, C., Blunder, M., Bauer, R., Gibbons, S., Bhakta, S., Bucar, F.: Synthesis and antibacterial evaluation of a new series of N-Alkyl-2-alkynyl/(E)-alkenyl-4-(1H)-quinolones. Molecules 17, 8217–8240 (2012)
Osman, K., Evangelopoulos, D., Basavannacharya, C., Gupta, A., McHugh, T.D., Bhakta, S., Gibbons, S.: An antibacterial from Hypericumacmosepalum inhibits ATP-dependent MurE ligase from Mycobacterium tuberculosis. Int. J. Antimicrob. 39, 124–129 (2012)
Tomasic, T., Sink, R., Zidar, N., Fic, A., Contreras-Martel, C., Dessen, A., Patin, D., Blanot, D., Müller-Premru, M., Gobec, S., Zega, A.: Dual inhibitor of MurD and MurE ligases from Escherichia coli and Staphylococcus aureus. ACS Med. Chem. Lett. 3, 626–630 (2012)
Perdih, A., Hrast, M., Barreteau, H., Gobec, S., Wolber, G., Solmajer, T.: Benzene-1, 3-dicarboxylic acid 2, 5-dimethylpyrrole derivatives as multiple inhibitors of bacterial Mur ligases (MurC–MurF). Bioorg. Med. Chem. 22, 4124–4134 (2014)
Perdih, A., Hrast, M., Pureber, K., Barreteau, H., Grdadolnik, S.G., Kocjan, D., Gobec, S., Solmajer, T., Wolber, G.: Furan-based benzene mono-and dicarboxylic acid derivatives as multiple inhibitors of the bacterial Mur ligases (MurC–MurF): experimental and computational characterization. J. Comput. Aided Mol. Des. 29, 541–560 (2015)
Guzman, J.D., Pesnot, T., Barrera, D.A., Davies, H.M., McMahon, E., Evangelopoulos, D., Mortazavi, P.N., Munshi, T., Maitra, A., Lamming, E.D., Angell, R.: Tetrahydroisoquinolines affect the whole-cell phenotype of Mycobacterium tuberculosis by inhibiting the ATP-dependent MurE ligase. J. Antimicrob. 70, 1691–1703 (2015)
Hrast, M., Rožman, K., Ogris, I., Skedelj, V., Patin, D., Sova, M., Barreteau, H., Gobec, S., Grdadolnik, S.G., Zega, A.: Evaluation of the published kinase inhibitor set to identify multiple inhibitors of bacterial ATP-dependent mur ligases. J. Enzym. Inhib. Med. Chem. 34, 1010–1017 (2019)
Meek, T.D., Johnson, K.A., Villafranca, J.J.: Escherichia coli glutamine synthetase. Determination of rate-limiting steps by rapid-quench and isotope partitioning experiments. Biochemistry 21, 2158–2167 (1982)
Meek, T.D., Villafranca, J.J.: Kinetic mechanism of Escherichia coli glutamine synthetase. Biochemistry 19, 5513–5519 (1980)
Midelfort, C.F., Rose, I.A.: A stereochemical method for detection of ATP terminal phosphate transfer in enzymatic reactions. Glutamine synthetase. J. Biol. Chem. 251, 5581–5587 (1976)
Shi, Y., Walsh, C.T.: Active-site mapping of Escherichia colid-Ala-d-Ala ligase by structure-based mutagenesis. Biochemistry 34, 2768–2776 (1995)
Fan, C., Moews, P.C., Walsh, C.T.: Vancomycin resistance: structure of d-alanine: d-alanine ligase at 2.3 Å resolution. Science 266, 439–443 (1994)
Wright, G.D., Walsh, C.T.: d-Alanyl-d-alanine ligases and the molecular mechanism of vancomycin resistance. Acc. Chem. Res. 25, 468–473 (1992)
Mullins, L.S., Zawadzke, L.E., Walsh, C.T., Raushel, F.M.: Kinetic evidence for the formation of d-alanyl phosphate in the mechanism of d-alanyl-d-alanine ligase. J. Biol. Chem. 265, 8993–8998 (1990)
Falk, P.J., Ervin, K.M., Volk, K.S., Ho, H.T.: Biochemical evidence for the formation of a covalent acyl-phosphate linkage between UDP-N-acetylmuramate and ATP in the Escherichia coli UDP-N-acetylmuramate: l-alanine ligase-catalyzed reaction. Biochemistry 35, 1417–1422 (1996)
Bouhss, A., Mengin-Lecreulx, D., Blanot, D., van-Heijenoort, J., Parquet, C.: Invariant amino acids in the Mur peptide synthetases of bacterial peptidoglycan synthesis and their modification by site-directed mutagenesis in the UDP-MurNAc: l-alanine ligase from Escherichia coli. Biochemistry 36, 11556–11563 (1997)
Vaganay, S., Tanner, M.E., van-Heijenoort, J.E., Blanot, D.: Study of the reaction mechanism of the d-glutamic acid-adding enzyme from Escherichia coli. Microb. Drug Resist. 2, 51–54 (1996)
Eveland, S.S., Pompliano, D.L., Anderson, M.S.: Conditionally lethal Escherichia coli murein mutants contain point defects that map to regions conserved among murein and folyl poly-γ-glutamate ligases: identification of a ligase superfamily. Biochemistry 36, 6223–6229 (1997)
Walsh, C., Bradley, M., Nadeau, K.: Molecular studies on trypanothione reductase, a target for antiparasitic drugs. Trends Biochem. 16, 305–309 (1991)
Katoh, M., Hiratake, J., Kato, H., Oda, J.I.: Mechanism-based inactivation of E. coli γ-glutamyl cysteine synthetase by phosphinic acid-and sulfoximine-based transition-state analogues. Bioorg. Med. Chem. Lett. 6, 1437–1442 (1996)
Hiratake, J., Kato, H., Oda, J.I.: Mechanism-based inactivation of glutathione synthetase by phosphinic acid transition-state analog. J. Am. Chem. Soc. 116, 12059–12060 (1994)
Kato, H., Tanaka, T., Yamaguchi, H., Hara, T., Nishioka, T., Katsube, Y., Oda, J.I.: Flexible loop that is novel catalytic machinery in a ligase. Atomic structure and function of the loopless glutathione synthetase. Biochemistry 33, 4995–4999 (1994)
Payne, D.J., Gwynn, M.N., Holmes, D.J., Pompliano, D.L.: Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 6, 29–40 (2007)
Lewis, K.: Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 12, 371–387 (2013)
Silver, L.L.: Does the cell wall of bacteria remain a viable source of targets for novel antibiotics? Biochem. Pharmacol. 71, 996–1005 (2006)
Nikaido, H.: Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 (2003)
Baum, E.Z., Crespo-Carbone, S.M., Klinger, A., Foleno, B.D., Turchi, I., Macielag, M., Bush, K.: A MurF inhibitor that disrupts cell wall biosynthesis in Escherichia coli. Antimicrob. Agents Chemother. 51, 4420–4426 (2007)
Baum, E.Z., Crespo-Carbone, S.M., Foleno, B.D., Simon, L.D., Guillemont, J., Macielag, M., Bush, K.: MurF inhibitors with antibacterial activity: effect on muropeptide levels. Antimicrob. Agents Chemother. 53, 3240–3247 (2009)
Kouidmi, I., Levesque, R.C., Paradis-Bleau, C.: The biology of Mur ligases as an antibacterial target. Mol. Microbiol. 94, 242–253 (2014)
Funding
We would like to thank the Science and Engineering Research Board (SERB), Government of India for the financial support (No. EMR/2016/002981).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saha, N., Azam, M.A. MurE inhibitors as antibacterial agents: a review. J Incl Phenom Macrocycl Chem 98, 127–136 (2020). https://doi.org/10.1007/s10847-020-01018-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-01018-6