Abstract
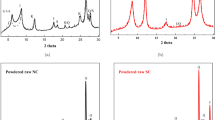
Zeolite was synthesized via alkaline fusion from the tailings dam sediments of a sand mine in southern Brazil. The sediments are composed of a mixture of illite, kaolinite, vermiculite, quartz and K-feldspar yielding a silty-loam texture. The extent of zeolite synthesis was tested under varied NaOH:sediment ratio, temperature and fusion time. At the NaOH:sediment ratio of 8:5 and fusion temperature of 600 °C, all minerals were dissolved and converted in faujasite, except a quartz residue of less than 5 wt%. The synthetic faujasite shows intergrown octahedral habit, grain size < 2 µm, specific surface area of 760 m2 g−1 and cation exchange capacity of 4.6 cmol kg−1. Experiments at lower NaOH:sediment ratio produced lower amounts of zeolite from 6:5 to 5:5 and 4:5, faujasite fraction declined from 73 to 50 wt%. At an NaOH:sediment ratio of 3:5, the faujasite fraction dropped to 0.7 wt%, below which no faujasite was formed. By decreasing temperature from 550 to 250 °C, a smooth decrease in faujasite formation was observed, from 64 to 29 wt%. A synthetic analogous of ferrierite appeared in the runs at 300 and 250 °C. Experiments using prolonged heating (up to 12 h) at low temperatures (300, 200 °C) and low NaOH:sediment ratio (3:5) did not enhance zeolite formation. Kaolinite and vermiculite were dissolved in all runs, while residual illite was ubiquitous and residual quartz was present in all experiments.






Similar content being viewed by others
References
Armbruster T, Gunter ME (2001) Crystal structures of natural zeolites. Rev Mineral Geochem 45:1–67
Awala H, Gilson JP, Retoux R, Boullay P, Goupil JM, Valtchev V, Mintova S (2015) Template-free nanosized faujasite-type zeolites. Nat Mater. https://doi.org/10.1038/NMAT4173
Baerlocher C, McCusker LB, Olson DH (2007) Atlas of zeolite framework types. Elsevier, Amsterdam
Bish DL, Von Dreele RB (1989) Rietveld refinement of non-hydrogen atomic positions in kaolinite. Clay Clay Miner 37:289–296
Castro PRS, Maia AAB, Angélica RS (2019) Study of thermal stability of faujasite zeolite synthesized from kaolin waste from the Amazon. Mater Res 22(5):1–7. https://doi.org/10.1590/1980-5373-MR-2019-0321
Coombs DS, Alberti A, Armbruster T, Artioli G, Colella C, Galli E, Grice JD, Liebau F, Mandarino JA, Minato H, Nickel EH, Passaglia E, Peacor DR, Quartieri S, Rinaldi R, Ross M, Sheppard RA, Tillmanns E, Vezzalini G (1997) Recommended nomenclature on zeolites: report of the subcommittee on zeolites of the International Mineralogical Association, commission on new minerals and mineral names. Can Miner 35:1571–1606
Fan Y, Zhang FS, Zhua J, Liu Z (2008) Effective utilization of waste ash from MSW and coal co-combustion power plant zeolite synthesis. J Hazard Mater 153:382–388
Flanigen EM (2001) Zeolites and molecular sieves: Na historical perspective. Stud Surf Sci Catal 137:11–35
Flanigen EM, Robert WB, Stephen TW (2010) Introduction. In: Kulprathipanja S (ed) Zeolites in industrial separation and catalysis. Wiley-VHC Verlag, New York, pp 1–26
Földvári M (2011) Handbook of thermogravimetric system of minerals and its use in geological practice. Geological Institute of Hungary, Budapest
Foleto EL, Hoffmann R, Hoffmann RS, Portugal UL Jr, Jahn SL (2005) Aplicabilidade das cinzas da casca de arroz. Quim Nova 28:1055–1060
Franus W, Wdowin M, Franus M (2014) Synthesis and characterization of zeolites prepared from industrial fly ash. Environ Monit Assess 186:5721–5729
Gotardi G, Gali E (1985) Natural zeolites. Springer, Berlin
Gramlich-Meier R, Gramlich V, Meier VM (1985) The crystal structure of the monoclinic variety of ferrierite. Am Mineral 70:619–623
Gražulis S, Chateigner D, Downs RT, Yokochi AFT, Quirós M, Lutterotti L, Manakova E, Butkus J, Moeck P, Le Bail A (2009) Crystallography open database—an open-access collection of crystal structures. J Appl Crystallogr 42:726–729
Grela A, Lach M, Mikula J, Hebda M (2016) Thermal analysis of the products of alkali activation of fly ash from CFB boilers. J Therm Anal Calorim 124:1609–1621
GSA Resources (2000) Cation-exchange capacity of zeolites. https://www.panaceo.hr/download/Cationexchange%2520capacity%2520of%2520zeolits.doc. Accessed 4 Mar 2015
Gualtieri AF (2000) Accuracy of XRPD QPA using the combined Rietveld-RIR method. J Appl Crys 33:267–278
Guan Q, Wu D, Lin Y, Chen X, Wang X, Li C, He S, Kong H (2009) Application of zeolitic material synthesized from thermally treated sediment to the removal of trivalent chromium from wastewater. J Hazard Mater 167:244–249
Halina M, Ramesh S, Yarmo MA, Kamarudin RA (2007) Non-hydrothermal synthesis of mesoprorous materials using sodium silicate from coal fly ash. Mater Chem Phys 101:344–351
Hamadi A, Nabih K (2018) Synthesis of zeolite materials using fly ash and oil shale ash and their applications in removing heavy metals from aqueous solutions. J Chem N Y. https://doi.org/10.1155/2018/6207910
Heller L (2019) Mining disasters and public health in Brazil: lessons (not) learned. Cad Saúde Pública 35(5):1–6. https://doi.org/10.1590/0102-311x00073619
Hendricks SB, Jefferson ME (1938) Crystal structures of vermiculite and mixed vermiculite-chlorite. Am Mineral 23:851–862
Hesse PR (1971) A textbook of soil chemical analysis. Chemical Publishing Co Inc, New York
IBRAM (Instituto Brasileiro de Mineração) (2012) Informações e Análises da Economia Mineral Brasileira. https://www.ibram.org.br/sites/1300/1382/00002806.pdf. Accessed 3 Oct 2018
Izidoro JC, Kim MC, Bellelli VF, Pane MC, Botelho Junior AB, Espinosa DCR, Tenório JAS (2019) Synthesis of zeolite A using the waste of iron mine tailings dam and its application for industrial effluent treatment. J Sustain Min. https://doi.org/10.1016/j.jsm.2019.11.001
Javed I, Mateen F, Rafique U, Tabassun N, Balkhair KS, Ashraf MA (2015) Synthesis of zeolite from marble powder waste: a greener approach and its application for the removal of inorganic metals from wastewater. Desalin Water Treat 57(22):10422–11043. https://doi.org/10.1080/19443994.2015.1033763
Johnson EBG, Arshad SE (2014) Hydrothermally synthesized zeolites based on kaolinite: a review. Appl Clay Sci 97–98:215–221
Kongkachuichay P, Lohsoontom P (2006) Phase diagram of zeolite synthesized from perlite and rice husk ash. Sci Asia 32:13–16
Li CY, Rees LVC (1986) The thermal stability of faujasites with different Si/Al ratios. Zeolites 6:60–65
Ma Y, Yan C, Alshameri A, Qiu X, Zhou C, Li D (2014) Synthesis and characterization of 13X zeolite from low-grade natural kaolin. Adv Powder Technol 25:495–499
Mallapur VP, Oubagaranadin JUK (2017) A brief review on the synthesis of zeolite from hazardous wastes. Trans Indian Ceram Soc 76(1):1–13
McCusker LB, Liebau F, Engelhardt G (2001) Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts. Pure Appl Chem 73(2):381–394
Mezni M, Hamzaoui A, Hamdi N, Srasra E (2011) Synthesis of zeolites from the low-grade Tunisian natural illite by two different methods. Appl Clay Sci 52:209–218
Ming DW, Allen ER (2001) Use of natural zeolites in agronomy, horticulture and environmental soil remediation. Rev Mineral Geochem 45:619–654
Moisés MP, Silva CTP, Meneguin JG, Girotto EM, Radovanovic E (2013) Synthesis of zeolite NaA from sugarcane bagasse ash. Mater Lett 108:243–246
Montégut G, Michelin L, Brendlé J, Lebeau B, Patarin J (2016) Ammonium and potassium removal from swine liquid manure using clinoptilolite, chabazite and faujasite zeolites. J Environ Manag 167:147–155. https://doi.org/10.1016/j.jenvman.2015.11.027
Moshoeshoe M, Nadiye-Tabbiruka MS, Obuseng V (2017) A review of the chemistry, structure, properties and applications of zeolites. Am J Mater Sci 7(5):196–221
Muñiz JGR, Ramírez AM, Robles JMA, Melo PG, Bocardo JCE, Martínez AM (2010) Synthesis and characterization of high silica zeolites from coal fly ash (CFA): two cases of zeolites synthesis from the same waste material. Lat Am Appl Res 40:323–328
Narasimhulu K, Gettu R, Babu G (2014) Beneficiation of natural zeolite through flash calcination for its use as a mineral admixture in concrete. J Mater Civ Eng 26:24–33
Patcharin W, Sriamporin K, Kanokkan A (2011) Utilization biomass from bagasse ash for phillipsite zeolite synthesis. Open J Adv Mater Res 383–390:4038–4042
Perez CAC, Resende NS, Salim VMM, Schmal M (2017) Water interaction in faujasite probed by in situ X-ray powder diffraction. Am J Phys Chem C 2(121):2755–2761. https://doi.org/10.1021/acs.jpcc.6b11111
Petrovic I, Navrotsky A (1997) Thermochemistry of Na-faujasites with varying Si/Al ratios. Microporous Mater 9:1–12
Querol X, Moreno N, Umaña JC, Alastuey A, Hernandez E, López-Soler A, Plana F (2002) Synthesis of zeolites from coal fly ash: an overview. Int J Coal Geol 50:413–423
Rayalu S, Meshram SU, Hasan MZ (2000) Highly crystalline faujasitic zeolites from fly ash. J Hazard Mater B77:123–131
Reiprich B, Weissenberger T, Schwieger W, Inayat A (2020) Layer-like FAU-type zeolites: a comparative view on different preparation routes. Front Chem Sci Eng. https://doi.org/10.1007/s11705-019-1883-3
Shiguemoto N, Hayashi H, Miyamura K (1993) Selective formation of Na-X zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. J Mater Sci 28:4781–4786
Shinzato MC, Montanheiro TJ, Andrade S, Carvalho FMS, Silva ML (2010) Synthesis of zeolites from sugarcane bagasse ash. In: Abstracts, 8th international conference of natural zeolites, Sofia, Bulgary
Silva Filho SH, Bieseki L, Dilva AR, Maia AAB, San Gil RAS, Pergher SBC (2015) Synthesis of zeolite A employing Amazon kaolin waste. Cerâmica 61:409–413
Stocker K, Ellersdorfer M, Lehner M, Raith JG (2017) Characterization and utilization of natural zeolites in technical applications. Berg Huettenmaenn Monatsh 162(4):142–147
Suguio K, Riccomini C, Sallun AEM, Sallun Filho W, Aronchi Neto P (2010) Provável significado geológico de idades LOE (Luminescência Opticamente Estimulada) da Formação Itaquaquecetuba, SP. Geologia USP 10(3):49–56
Terzano R, D’Alessandro C, Spagnuolo M, Romagnoli M, Medic L (2015) Facile zeolite synthesis from municipal glass and aluminum solid wastes. Clean: Soil, Air, Water 43(1):133–140
Thuadaij P, Nuntiya A (2012) Preparation and characterization of faujasite using fly ash and amorphous silica from rice husk ash. Procedia Eng 32:1026–1032. https://doi.org/10.1016/j.proeng.2012.02.049
Toby BH (2006) R factors in Rietveld analysis: how good is good enough? Powder Diffr 21(1):67–70
Toby BH, Von Dreele RB (2013) GSAS II: the genesis of a modern open-source all purpose crystallography sotware package. J Appl Crystallogr 46:544–549
USGS (United States Geological Survey, 2019). Zeolite—mineral commodity summaries. https://www.usgs.gov/centers/nmic/zeolites-statistics-and-information. Accessed 12 Dec 2019
Verboekend D, Nuttens N, Loxus R, Van Aelst J, Verolme P, Groen JC, Pérez-Ramírez J, Sels BJ (2016) Synthesis, characterisation, and catalytic evaluation of hierarchical faujasite zeolites: milestones, challenges, and future directions. Chem Soc Rev 45:3331–3352
Wajima T, Munakata K, Ikegami Y (2010) Conversion of waste sandstone cake into crystalline zeolite X using alkali fusion. Mater Trans 51(5):849–854
Worathanakul P, Mothong P, Engkawara P (2013) Fe2O3–SiO2 nanocomposite derived from bagasse ash for Cr(VI) removal. J Biobased Mater 7:219–222
Zendelska A, Golomeova M, Golomeov B, Krste B (2018) Removal of lead ions from acid aqueous solutions and acid mine drainage using zeolite-bearing tuff. Arch Environ Prot 44(1):87–96
Acknowledgements
The authors acknowledge the XRD data kindly provided by L. Gobbo (Malvern Panalytical) and the XRF analysis by M. Pecchio (ABCP São Paulo). We thank R. Contessoto for support in the acquisition of SEM images. The study was sponsored by a Fapesp Grant (#2014/15748-3) to M. T. A. G. Yogi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Andrade, F.R.D., Yogi, M.T.A.G., Gomes, E.B. et al. Extent of zeolite synthesis via alkaline fusion from tailings dam sediments. Environ Earth Sci 79, 379 (2020). https://doi.org/10.1007/s12665-020-09118-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-020-09118-9