Abstract
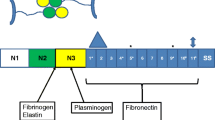
Streptococcus parasanguinis is a primary colonizer of dental plaque and an opportunistic pathogen for subacute endocarditis. A putative fibronectin binding protein (Spaf_1409) that lacks both an N-terminal signal peptide and a C-terminal cell wall-anchoring motif was identified from the S. parasanguinis FW213 genome. Spaf_1409 was abundantly present in the cytoplasm and also was found in the cell wall preparation and culture supernatant. By using an isogenic mutant strain, MPH4, Spaf_1409 was found to mediate the binding of S. parasanguinis FW213 to fibronectin. Inactivation of Spaf_1409 did not significantly alter the mass of static biofilm, but reduced the resistance of S. parasanguinis against the shearing force in a flow cell biofilm system, resulting in scattered biofilm. The mortality in Galleria mellonella larvae infected with MPH4 was higher than in those infected with wild-type S. parasanguinis. However, fewer viable bacterial cells were recovered from larvae infected with MPH4, compared to those infected with wild-type S. parasanguinis, up to 42 h post infection, suggesting that the infection by MPH4, but not the growth, was responsible for the elevated mortality. The phagocytic analysis using flow cytometry indicated that Spaf_1409 participates in the recognition of S. parasanguinis FW213 by RAW264.7 macrophages, suggesting that inactivation of Spaf_1409 intensified the immune responses in larvae, leading to larval death. Taken together, the data indicate that Spaf_1409 plays different roles in the development of dental biofilm and in systemic infections.





Similar content being viewed by others
References
Henderson B, Nair S, Pallas J, Williams MA (2011) Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol Rev 35(1):147–200. https://doi.org/10.1111/j.1574-6976.2010.00243.xFMR243[pii]
Joh D, Wann ER, Kreikemeyer B, Speziale P, Hook M (1999) Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol 18(3):211–223
Chhatwal GS (2002) Anchorless adhesins and invasins of Gram-positive bacteria: a new class of virulence factors. Trends Microbiol 10(5):205–208. https://doi.org/10.1016/s0966-842x(02)02351-x
Josse J, Laurent F, Diot A (2017) Staphylococcal adhesion and host cell invasion: fibronectin-binding and other mechanisms. Front Microbiol 8:2433. https://doi.org/10.3389/fmicb.2017.02433
Yamaguchi M, Terao Y, Kawabata S (2013) Pleiotropic virulence factor-Streptococcus pyogenes fibronectin-binding proteins. Cell Microbiol 15(4):503–511. https://doi.org/10.1111/cmi.12083
Holmes AR, McNab R, Millsap KW, Rohde M, Hammerschmidt S, Mawdsley JL, Jenkinson HF (2001) The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence. Mol Microbiol 41(6):1395–1408
Kawabata S, Kunitomo E, Terao Y, Nakagawa I, Kikuchi K, Totsuka K, Hamada S (2001) Systemic and mucosal immunizations with fibronectin-binding protein FBP54 induce protective immune responses against Streptococcus pyogenes challenge in mice. Infect Immun 69(2):924–930. https://doi.org/10.1128/IAI.69.2.924-930.2001
Musyoki AM, Shi Z, Xuan C, Lu G, Qi J, Gao F, Zheng B, Zhang Q, Li Y, Haywood J, Liu C, Yan J, Shi Y, Gao GF (2016) Structural and functional analysis of an anchorless fibronectin-binding protein FBPS from Gram-positive bacterium Streptococcus suis. Proc Natl Acad Sci USA 113(48):13869–13874. https://doi.org/10.1073/pnas.1608406113
Hanski E, Caparon M (1992) Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci U S A 89(13):6172–6176
Molinari G, Talay SR, Valentin-Weigand P, Rohde M, Chhatwal GS (1997) The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun 65(4):1357–1363
Okada N, Tatsuno I, Hanski E, Caparon M, Sasakawa C (1998) Streptococcus pyogenes protein F promotes invasion of HeLa cells. Microbiology 144(Pt 11):3079–3086
Burke FM, Di Poto A, Speziale P, Foster TJ (2011) The A domain of fibronectin-binding protein B of Staphylococcus aureus contains a novel fibronectin binding site. FEBS J 278(13):2359–2371. https://doi.org/10.1111/j.1742-4658.2011.08159.x
Roche FM, Downer R, Keane F, Speziale P, Park PW, Foster TJ (2004) The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J Biol Chem 279(37):38433–38440. https://doi.org/10.1074/jbc.M402122200M402122200[pii]
Fowler T, Wann ER, Joh D, Johansson S, Foster TJ, Hook M (2000) Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell beta1 integrins. Eur J Cell Biol 79(10):672–679. https://doi.org/10.1078/0171-9335-00104
Massey RC, Kantzanou MN, Fowler T, Day NP, Schofield K, Wann ER, Berendt AR, Hook M, Peacock SJ (2001) Fibronectin-binding protein A of Staphylococcus aureus has multiple, substituting, binding regions that mediate adherence to fibronectin and invasion of endothelial cells. Cell Microbiol 3(12):839–851
Sinha B, Herrmann M (2005) Mechanism and consequences of invasion of endothelial cells by Staphylococcus aureus. Thromb Haemost 94(2):266–277
Shinji H, Yosizawa Y, Tajima A, Iwase T, Sugimoto S, Seki K, Mizunoe Y (2011) Role of fibronectin-binding proteins A and B in in vitro cellular infections and in vivo septic infections by Staphylococcus aureus. Infect Immun 79(6):2215–2223. https://doi.org/10.1128/IAI.00133-11
Pracht D, Elm C, Gerber J, Bergmann S, Rohde M, Seiler M, Kim KS, Jenkinson HF, Nau R, Hammerschmidt S (2005) PavA of Streptococcus pneumoniae modulates adherence, invasion, and meningeal inflammation. Infect Immun 73(5):2680–2689
Noske N, Kammerer U, Rohde M, Hammerschmidt S (2009) Pneumococcal interaction with human dendritic cells: phagocytosis, survival, and induced adaptive immune response are manipulated by PavA. J Immunol 183(3):1952–1963. https://doi.org/10.4049/jimmunol.0804383
Kolenbrander PE (2000) Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol 54:413–437. https://doi.org/10.1146/annurev.micro.54.1.413
Douglas CW, Heath J, Hampton KK, Preston FE (1993) Identity of viridans streptococci isolated from cases of infective endocarditis. J Med Microbiol 39(3):179–182
Sadjadi SA, Ali H (2011) Streptococcus parasanguis peritonitis: report of a case and review of the literature. Perit Dial Int 31(5):603–604. https://doi.org/10.3747/pdi.2011.0001131/5/603[pii]
Lowrance JH, Baddour LM, Simpson WA (1990) The role of fibronectin binding in the rat model of experimental endocarditis caused by Streptococcus sanguis. J Clin Invest 86(1):7–13. https://doi.org/10.1172/JCI114717
Loo CY, Corliss DA, Ganeshkumar N (2000) Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J Bacteriol 182(5):1374–1382
Bartolome B, Jubete Y, Martinez E, de la Cruz F (1991) Construction and properties of a family of pACYC184-derived cloning vectors compatible with pBR322 and its derivatives. Gene 102(1):75–78. https://doi.org/10.1016/0378-1119(91)90541-i
Rubens CE, Heggen LM (1988) Tn916 delta E: a Tn916 transposon derivative expressing erythromycin resistance. Plasmid 20(2):137–142. https://doi.org/10.1016/0147-619x(88)90016-9
Chen YY, Weaver CA, Mendelsohn DR, Burne RA (1998) Transcriptional regulation of the Streptococcus salivarius 57.I urease operon. J Bacteriol 180(21):5769–5775
Giomarelli B, Visai L, Hijazi K, Rindi S, Ponzio M, Iannelli F, Speziale P, Pozzi G (2006) Binding of Streptococcus gordonii to extracellular matrix proteins. FEMS Microbiol Lett 265(2):172–177. https://doi.org/10.1111/j.1574-6968.2006.00479.x
Wu H, Mintz KP, Ladha M, Fives-Taylor PM (1998) Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol Microbiol 28(3):487–500
Liang X, Chen YY, Ruiz T, Wu H (2011) New cell surface protein involved in biofilm formation by Streptococcus parasanguinis. Infect Immun 79(8):3239–3248. https://doi.org/10.1128/IAI.00029-11
Chen YY, Chen YY, Hung JL, Chen PM, Chia JS (2016) The GlnR regulon in Streptococcus mutans is differentially regulated by GlnR and PmrA. PLoS ONE 11(7):e0159599. https://doi.org/10.1371/journal.pone.0159599
O'Toole GA, Kolter R (1998) Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28(3):449–461. https://doi.org/10.1046/j.1365-2958.1998.00797.x
Geng J, Huang SC, Chen YY, Chiu CH, Hu S, Chen YM (2018) Impact of growth pH and glucose concentrations on the CodY regulatory network in Streptococcus salivarius. BMC Genomics 19(1):386. https://doi.org/10.1186/s12864-018-4781-z
Chen YY, Shieh HR, Lin CT, Liang SY (2011) Properties and construction of plasmid pFW213, a shuttle vector with the oral Streptococcus origin of replication. Appl Environ Microbiol 77(12):3967–3974. https://doi.org/10.1128/AEM.02828-10
Sokolovska A, Becker CE, Stuart LM (2012) Measurement of phagocytosis, phagosome acidification, and intracellular killing of Staphylococcus aureus. Curr Protoc Immunol Chap 14(Unit14):30. https://doi.org/10.1002/0471142735.im1430s99
Chen YY, Shieh HR, Chang YC (2013) The expression of the fim operon is crucial for the survival of Streptococcus parasanguinis FW213 within macrophages but not acid tolerance. PLoS ONE 8(6):e66163. https://doi.org/10.1371/journal.pone.0066163
Wu H, Fives-Taylor PM (1999) Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1, of Streptococcus parasanguis. Mol Microbiol 34(5):1070–1081
Froeliger EH, Fives-Taylor P (2001) Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation. Infect Immun 69(4):2512–2519. https://doi.org/10.1128/IAI.69.4.2512-2519.2001
Henderson B, Martin AC (2014) Protein moonlighting: a new factor in biology and medicine. Biochem Soc Trans 42(6):1671–1678. https://doi.org/10.1042/BST20140273
Henderson B, Martin A (2013) Bacterial moonlighting proteins and bacterial virulence. Curr Top Microbiol Immunol 358:155–213. https://doi.org/10.1007/82_2011_188
Dallo SF, Kannan TR, Blaylock MW, Baseman JB (2002) Elongation factor Tu and E1 beta subunit of pyruvate dehydrogenase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Mol Microbiol 46(4):1041–1051
Flock M, Flock JI (2001) Rebinding of extracellular adherence protein Eap to Staphylococcus aureus can occur through a surface-bound neutral phosphatase. J Bacteriol 183(13):3999–4003. https://doi.org/10.1128/JB.183.13.3999-4003.2001
Hussain M, Haggar A, Peters G, Chhatwal GS, Herrmann M, Flock JI, Sinha B (2008) More than one tandem repeat domain of the extracellular adherence protein of Staphylococcus aureus is required for aggregation, adherence, and host cell invasion but not for leukocyte activation. Infect Immun 76(12):5615–5623. https://doi.org/10.1128/IAI.00480-08
Yonemoto K, Chiba A, Sugimoto S, Sato C, Saito M, Kinjo Y, Marumo K, Mizunoe Y (2019) Redundant and distinct roles of secreted protein Eap and cell wall-anchored rrotein SasG in biofilm formation and pathogenicity of Staphylococcus aureus. Infect Immun 87(4):e00894. https://doi.org/10.1128/IAI.00894-18
Browne N, Heelan M, Kavanagh K (2013) An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence 4(7):597–603. https://doi.org/10.4161/viru.25906
Tsai CJ, Loh JM, Proft T (2016) Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 7(3):214–229. https://doi.org/10.1080/21505594.2015.1135289
Mak P, Zdybicka-Barabas A, Cytrynska M (2010) A different repertoire of Galleria mellonella antimicrobial peptides in larvae challenged with bacteria and fungi. Dev Comp Immunol 34(10):1129–1136. https://doi.org/10.1016/j.dci.2010.06.005
Harding CR, Schroeder GN, Reynolds S, Kosta A, Collins JW, Mousnier A, Frankel G (2012) Legionella pneumophila pathogenesis in the Galleria mellonella infection model. Infect Immun 80(8):2780–2790. https://doi.org/10.1128/IAI.00510-12
Cole RM, Calandra GB, Huff E, Nugent KM (1976) Attributes of potential utility in differentiating among "group H" streptococci or Streptococcus sanguis. J Dent Res 55:A142–153
Acknowledgements
This work was supported by the Chang Gung Memorial Hospital of Taiwan, Grant CMRPD1J0041-3 and 1J0301 to Y. M. Chen. We thank R. Faustoferri for review of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: YMC and PL. Performed the experiments: PL and PT. Analyzed the data: YMC, PL, and PT. Contributed reagents/materials/analysis tools: CC. Wrote the paper: YMC and PL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, YY.M., Lu, PS., Tsai, PH. et al. Functional Analysis of a Fibronectin Binding Protein of Streptococcus parasanguinis FW213. Curr Microbiol 77, 3430–3440 (2020). https://doi.org/10.1007/s00284-020-02152-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02152-7