Abstract
The transformative effect of oxygen on conventional x-ray radiotherapy has long been known, with the presence of molecular oxygen boosting treatment efficacy multi-fold. This effect is present too in charged particle therapy, but the boosting potential decreases with increasing linear energy transfer of the incident radiation. With particle modalities such as proton therapy becoming common-place and emerging technologies like carbon-ion therapy on the horizon, it is pertinent to address the additive dose boost gained from molecular oxygen with high energy radiation, and the implications of this for dose planning. This work establishes an empirical model for oxygen enhancement across the radiotherapy energy spectrum, and discusses implications of this for therapy.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The substantial benefits of molecular oxygen on treatment response has long been known and clinically exploited. For conventional x-ray therapy, the presence of oxygen can boost cell kill by up to a factor of approximately three (Evans and Koch 2003, Vaupel and Mayer 2007, Wilson and Hay 2011, Grimes et al 2017). This improved kill with oxygen is known as the oxygen enhancement ratio (OER), typically defined as the ratio of cell kill under highly oxygenated conditions relative to the cell kill under anoxia (Hall and Giaccia 2006). The non-linear quasi-hyperbolic variation of OER with oxygen partial pressure is a consequence of the Oxygen Fixation Hypothesis (OFH, (Bertout et al 2008)). Under the OFH model, direct damage by hydroxyl radicals and related products is considered relatively easy to chemically reverse. However, when these radical produces encounter oxygen molecules, they have the capacity to 'fix' damage, preventing easy chemical repair and resulting in improved kill. This has been described mechanistically in recent years (Grimes and Partridge 2015), and as a strictly chemical process manifests in all cell types.
While this is well-understood for x-ray radiation, OER changes markedly as a function of Linear Energy Transfer (LET) in charged particle therapy, decreasing to unity at very high LETs (Hall and Giaccia 2006). The likely reason for this is that under increasing LET, direct damage to DNA due to interactions with charged particles becomes the dominant process, and the contribution of oxygen fixation towards cell-kill diminishes as direct kill increases (Grimes et al 2017). A major appeal of charged particle therapy is that high doses can be very effectively delivered to chosen regions, whilst largely sparing healthy tissue. At the target site, direct kill typically suffices so that oxygen enhancement is not required. However, LET is non-zero along the particle range, and can have interactions with oxygen which would increase the cell-kill along the particle range, potentially resulting in increased damage to healthy physoxic cells in many instances. To the author's knowledge, this is a facet of particle therapy that has not yet been extensively studied, and may have ramifications for treatment. In this work, we establish an empirical model for this process, and explore the implications of this effect on therapy.
2. Model derivation
For the interaction of x-ray photons, a model has been previously derived (Grimes and Partridge 2015) which quantifies the relationship between OER and oxygen tension, given by

where p is the oxygen partial pressure. The rate constant ϕ can be theoretically derived from the thermal velocity of oxygen molecules in water, the mean-free path of oxygen molecules and the probability of an interaction between oxygen molecule and ionized DNA, and has previously been measured to be 0.26 ± 0.02 mmHg−1 (Grimes and Partridge 2015). Oxygen partial pressure here is measured in units of mmHg, in line with historical medical convention, but this can readily be converted to other units if required (Grimes et al 2014). In this model, φO denotes the fraction of cells killed under oxic conditions and φD those killed by direct interaction, which have been experimentally determined previously for x-ray photons, with ϕ having no known dependence on LET. Here we derive a method to empirically estimate how this will vary under changing LET. We initially assume that cell kill events are independent, and direct cell kill events (when a charged particle impinges directly on DNA) occur at a rate χD per particle LET. Indirect cell kill occurs where a charged particle means an oxygen molecule under OFH, at a rate χI per particle LET. We initially assume these two processes are independent for simplicity. In this case, the probability of these processes occuring and resulting in a cell-kill event are given by Poisson statistics as


where L is LET in keV/µm. The number of cells killed is a function of of particle fluence along the track length, and the LET specific cell kill probability. This complex expression however does not have to be explicitly calculated here. Instead, we note that the fraction of cells killed under oxic conditions over direct kill is congruent to the fraction of indirect kill probability over direct, so that
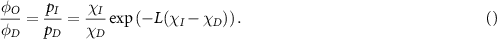
In this case, we may re-write equation (1) empirically in terms of LET and oxygen partial pressure as
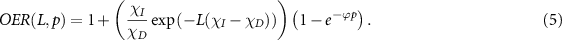
2.1. Parameter fitting
For this to be useful, it is important that the empirical constants for direct and indirect kill, χD and χI, respectively, can be estimated. To achieve these estimates, OER measurements were taken from previously published data from 13 different cell-lines, comprising mammalian and human cell lines, both cancerous and non-cancerous, originally measured in various investigations between 1965 and 2009. This data has previously been published and curated in this journal, and full details are available in table 1 of (Wenzl and Wilkens 2011). A summary of this data is given in table 1 here. Equation (5) was then fitted to this data and the best fit parameters ascertained from this. In addition, as this data spans several decades and different investigations, data taken at the same LET was combined and points weighted. This weighted fit was taken to minimise potential errors introduced by data outliers.
Table 1. Cell line OER data.
| Cell line | Type | Number of studies |
|---|---|---|
| V-79: Chinese Hamster Lung Cell | Mammalian cell (non-cancer) | 18 |
| CHO: Chinese Hamster Ovary Cell | Mammalian cell (non-cancer) | 10 |
| L2 - Rat Pulmonary | Mammalian cell (non-cancer) | 1 |
| R-AT - Rat Fibroblast | Mammalian cell (non-cancer) | 1 |
| Rat Embryo | Mammalian cell (non-cancer) | 1 |
| EMT6: Murine mammary carcinoma | Mammalian cell (cancer) | 8 |
| P388 Murine leukaemia | Mammalian cell (cancer) | 3 |
| FSa - Murine fibrosarcoma | Mammalian cell (cancer) | 2 |
| 9 L: Rat malignant glioma | Mammalian cell (cancer) | 6 |
| HeLa: Human cervical cancer | Human cell (cancer) | 3 |
| T-1: HeLa derived cell line | Human cell (cancer) | 14 |
| HSG: HeLa derived cell line | Human cell (cancer) | 2 |
| SCC - Squamous carcinoma | Human cell (cancer) | 2 |
2.2. OER variation with range for proton and carbon-ion therapy
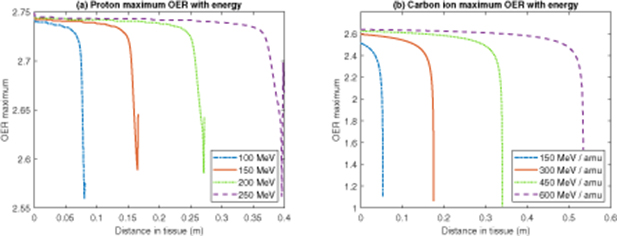
The simple model derived here can be used to ascertain potential ramifications for cell-kill through tissue with charged particles. An attractive property of charged particle therapies is that they can be used to minimise radiation dose to healthy tissue as they traverse through it, delivering the vast majority of their energy at target. While relatively constant in healthy tissue, tumour oxygenation varies strikingly within tumour tissue. Solid tumours themselves frequently have extensive pockets of hypoxia and necrosis. It's worth considering how these factors might impact the delivery of therapy. Maximum OER was calculated in this work for different particle energies. For protons, LET and range for pencil beams of 100 MeV, 150 MeV, 200 MeV, and 250 MeV were calculated using the Monte Carlo N-Particle extended (MCNPX) v2.7.0 package (Pelowitz et al 2011), where LET profiles were reported at 1 mm intervals using surface tallies (MCNPX type F2, binned into 1000 subdivisions using the E card, modified to tally unrestricted stopping power by including the LET flag on an FT card). For carbon-ions, a previously derived analytical LET / Range method was employed for particles of 150 MeV / a.m.u, 300 MeV / a.m.u, 450 MeV / a.m.u, and 600 MeV / a.m.u (Grimes et al 2017).
3. Results
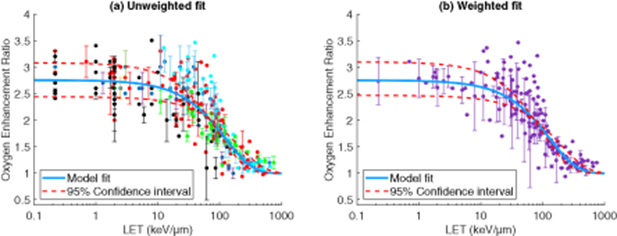
Model fitting results are given in table 2, and shown over the various data sets in figure 1. The weighted fit achieved a higher co-efficient of determination than the unweighted approach. Weighted fit values were used to estimate the maximum OER achievable with protons and carbon ions of varying energy along their track, as depicted in figure 2.
Figure 1. (a) Model fit to raw data (colour of data points indicate investigation from whence data was taken) (b) Model fit to weighted data, with multiple measurements at the same LET averaged and weighted, with error bars of 1 standard deviation shown.
Download figure:
Standard image High-resolution imageFigure 2. Maximum OER with range for (a) Protons of varying energy (b) Carbon ions of varying energy.
Download figure:
Standard image High-resolution imageTable 2. Fit parameters / measured co-efficient of determination R2.
| Fit process | Parameter | Fitted value (95% confidence interval) |
|---|---|---|
| Unweighted | χD | 1.049 × 10−2 (9.595 × 10−3 - 1.138 × 10−2 ) µm/keV |
| Unweighted | χI | 1.84 × 10−2 (1.685 × 10−2 - 1.994 × 10−2) µm/keV |
| Unweighted | R2 | 0.704 2 |
| Weighted | χD | 1.006 × 10−2 (9.157 × 10−3 - 1.096 × 10−2 ) µm/keV |
| Weighted | χI | 1.761 × 10−2 (1.605 × 10−2 - 1.918 × 10−2) µm/keV |
| Weighted | R2 | 0.838 7 |
4. Discussion
The model derived herein suggests that for protons even up to relatively high energy, the maximum OER is still close to the theoretical maximum, and typically above 2.74 until right before the terminus of the track. Carbon ions had lower maximum OERs than protons, due to their significantly higher LET along the path length, which also fell rapidly on approach the terminal end of the particle's path. However, along most of the path, OER remained close to maximum value, typically over 2.5 for the energies considered in this work.
This has potential clinical implications - as charged particles traverse physoxic tissue, their effective cell kill is amplified by the oxygen enhancement ratio possible. As healthy tissue is typically well-oxygenated, then the potential for increased cell-kill of healthy tissue along the path should be considered. OER for different LET can be explicitly taken from the model outlined here, which is itself a multiplier for projected cell-kill. This suggests that without correction, it is likely that dose to healthy tissue would be under-estimated.
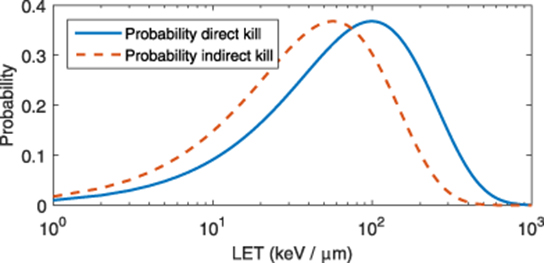
The model outlined here is empirical, and not explicitly mechanistic. One might reasonably enquire as to whether the derived parameters are physically realistic, or simply phenomenological. It's worth examining the parameters χD and χI to ascertain whether they have any physical significance. The probability functions outlined in equations (2) and 3 are plotted in figure 3. Function maximums occur where  and
and  , respectively, so that the values of L which maximises pD and pI are
, respectively, so that the values of L which maximises pD and pI are  and
and  . This yields a maximum probability for direct kill at 99.4 keV / µ m (95% confidence interval: 91.2 -109.2 keV / µ m). This agrees well with literature values for optimal cell-kill of 100 keV / µ m ((Joiner and Van der Kogel 2009), (Hasan 2019)).
. This yields a maximum probability for direct kill at 99.4 keV / µ m (95% confidence interval: 91.2 -109.2 keV / µ m). This agrees well with literature values for optimal cell-kill of 100 keV / µ m ((Joiner and Van der Kogel 2009), (Hasan 2019)).
Figure 3. Plot of kill probability with LET for direct and indirect kill.
Download figure:
Standard image High-resolution imageIt is important to note that there are limitations to such a model - because it is not mechanistic, there are other physical processes that might result in more nuanced effects at certain energies. Nor does this approach explicitly address the impacts of varying oxygen on predicted α / β ratios, which can be empirically estimated by other means (Wenzl and Wilkens 2011). The chief benefit of this simple model is that it provides a basis for rapid estimation of likely OER for different LET and partial pressure, generalising previous work (Grimes and Partridge 2015) on photons to account for charged particles. This makes it useful as a basis for simulations of dose to healthy and tumour tissue, or a starting point for more mechanistic investigation of oxygen and charged particle interactions on therapy outcome.
Acknowledgment
The author would like to profoundly thank Drs Tatiana Wenzl and Jan J Wilkens for kindly sharing the raw OER data referred to in this work.