Abstract
Silver nanoparticle (Ag NP)–coated carbon quantum dot (CQD) core-shell-structured nanocomposites (CQD@Ag NCs) were developed for fluorescent imaging of intracellular superoxide anion (O2•−). The morphology of CQD@Ag NCs was investigated by transmission electron microscopy, and the composition was characterized by X-ray diffraction and X-ray photoelectron spectroscopy. CQDs display blue fluorescence with excitation/emission maxima at 360/440 nm, and the fluorescence was quenched by Ag NPs in CQD@Ag NCs. In the presence of O2•−, Ag NPs were oxide-etched and the fluorescence of CQDs was recovered. A linearity between the relative fluorescence intensity and O2•− solution concentration within the range 0.6 to 1.6 μM was found, with a detection limit of 0.3 μM. Due to their high sensitivity, selectivity, and low cytotoxicity, the as-synthesized CQD@Ag NCs have been successfully applied for imaging of O2•− in MCF-7 cells during the whole process of autophagy induced by serum starvation. In our perception, the developed method provides a cost-effective, sensitive, and selective tool in bioimaging and monitoring of intracellular O2•− changes, and is promising for potential biological applications.

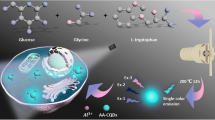
Illustration of the synthesis of carbon quantum Dot@Silver nanocomposites (CQD@Ag NCs), and CQD@Ag NCs as a “turn-on” nanoprobe for fluorescent imaging of intracellular superoxide anion






Similar content being viewed by others
References
Peng F, Xu T, Wu F, Ma C, Liu Y, Li J, Zhao B, Mao C (2017) Novel biomimetic enzyme for sensitive detection of superoxide anions. Talanta 174:82–91. https://doi.org/10.1016/j.talanta.2017.05.028
Gorrini C, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12(12):931–947. https://doi.org/10.1038/nrd4002
Labib M, Sargent EH, Kelley SO (2016) Electrochemical methods for the analysis of clinically relevant biomolecules. Chem Rev 116(16):9001–9090. https://doi.org/10.1021/acs.chemrev.6b00220
Liu X, Ran MM, Guoan XH, Xue ZH, Lu XQ (2018) A sensitively non-enzymatic amperometric sensor and its application in living cell superoxide anion radical detection. Talanta 186:248–255. https://doi.org/10.1016/j.talanta.2018.04.067
Li Y, Zhang H, Cai X, Zhao H, Magdassi S, Lan M (2019) Electrochemical detection of superoxide anions in HeLa cells by using two enzyme-free sensors prepared from ZIF-8-derived carbon nanomaterials. Microchim Acta 186(6):370. https://doi.org/10.1007/s00604-019-3473-y
Zheng J, Wang B, Jin Y, Weng B, Chen J (2019) Nanostructured MXene-based biomimetic enzymes for amperometric detection of superoxide anions from HepG2 cells. Microchim Acta 186(2):95. https://doi.org/10.1007/s00604-018-3220-9
Ying W, Lei MQ, Chen LL, Liu ZY, Bao YS (2018) FePO4 embedded in nanofibers consisting of amorphous carbon and reduced graphene oxide as an enzyme mimetic for monitoring superoxide anions released by living cells. Microchim Acta 185(2):140. https://doi.org/10.1007/s00604-018-2691-z
Yang H, Hou J, Wang Z, Zhang T, Xu C (2018) An ultrasensitive biosensor for superoxide anion based on hollow porous PtAg nanospheres. Biosens Bioelectron 117:429–435. https://doi.org/10.1016/j.bios.2018.06.034
Zhang H, Cai X, Zhao H, Sun W, Wang Z, Lan M (2019) Enzyme-free electrochemical sensor based on ZIF-67 for the detection of superoxide anion radical released from SK-BR-3 cells. J Electroanal Chem 855:113653. https://doi.org/10.1016/j.jelechem.2019.113653
Liu Y, Liu X, Liu Y, Liu G, Ding L, Lu X (2017) Construction of a highly sensitive non-enzymatic sensor for superoxide anion radical detection from living cells. Biosens Bioelectron 90:39–45. https://doi.org/10.1016/j.bios.2016.11.015
Wildgoose GG, Banks CE, Compton RG (2006) Metal nanoparticles and related materials supported on carbon nanotubes: methods and applications. Small 2(2):182–193. https://doi.org/10.1002/smll.200500324
Liu Y, Wei H, Jiang X, Guo H, Liu X (2018) Synthesis of metal–organic frameworks derived nanocomposites for superoxide anion radical sensing and cell monitoring upon oxidative stress. J Electroanal Chem 820:51–59. https://doi.org/10.1016/j.jelechem.2018.04.068
Li N, Wang H, Xue M, Chang C, Chen Z, Zhuo L, Tang B (2012) A highly selective and sensitive nanoprobe for detection and imaging of the superoxide anion radical in living cells. Chem Commun 48(19):2507–2509. https://doi.org/10.1039/c2cc16376d
Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS (2006) Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. PNAS 103(41):15038–15043. https://doi.org/10.1073/pnas.0601945103
Henderson LM, Chappell JB (1993) Dihydrorhodamine 123: a fluorescent probe for superoxide generation? Eur J Biochem 217(3):973–980. https://doi.org/10.1111/j.1432-1033.1993.tb18328.x
Gao X, Ding C, Zhu A, Tian Y (2014) Carbon-dot-based ratiometric fluorescent probe for imaging and biosensing of superoxide anion in live cells. Anal Chem 86(14):7071–7078. https://doi.org/10.1021/ac501499y
Zhou Y, Ding J, Liang T, Abdel-Halim ES, Jiang L, Zhu JJ (2016) FITdoped rattle-type silica colloidal particleatiometric fluorescent sensor for biosensing and imaging of superoxide anion. ACS Appl Mater Interfaces 8:6423–6430. https://doi.org/10.1021/acsami.6b01031
Chen Z, Li J, Chen X, Cao J, Zhang J, Min Q, Zhu JJ (2015) Single gold@silver nanoprobes for real-time tracing the entire autophagy process at single-cell level. J Am Chem Soc 137(5):1903–1908. https://doi.org/10.1021/ja5112628
Lu D, Zhou L, Wang R, Zhang X-B, He L, Zhang J, Hu X, Tan W (2017) A two-photon fluorescent probe for endogenous superoxide anion radical detection and imaging in living cells and tissues. Sensors Actuators B Chem 250(2017):259–266. https://doi.org/10.1016/j.snb.2017.04.041
Yuan L, Lin W, Zheng K, Zhu S (2013) FRET-based small-molecule fluorescent probes: rational design and bioimaging applications. Acc Chem Res 46(7):1462–1473. https://doi.org/10.1021/ar300273v
Chen L, Cho MK, Wu D, Kim HM, Yoon J (2019) Two-photon fluorescence probe for selective monitoring of superoxide in live cells and tissues. Anal Chem 91(22):14691–14696. https://doi.org/10.1021/acs.analchem.9b03937
Xi Z, Yuan F, Wang Z, Li S, Fan L (2018) Highly efficient and stable full-color random lasing emission based on carbon quantum dots. Acta Chim Sin 76(6):460. https://doi.org/10.6023/A18020048
Singh V, Rawat K, Mishra S, Baghel T, Fatima S (2018) Biocompatible fluorescent carbon quantum dots prepared from beetroot extract for in vivo live imaging in C-elegans and BALB/c mice. J Mater Chem B 6(20):3366–3371. https://doi.org/10.1039/c8tb00503f
Wang WJ, Xia JM, Feng J, He MQ, Chen ML, Wang JH (2016) Green preparation of carbon dots for intracellular pH sensing and multicolor live cell imaging. J Mater Chem B 4(44):7130–7137. https://doi.org/10.1039/c6tb02071b
Sharma V, Kaur N, Tiwari P, Mobin SM (2018) Full color emitting fluorescent carbon material as reversible pH sensor with multicolor live cell imaging. J Photochem Photobiol B 182:137–145. https://doi.org/10.1016/j.jphotobiol.2018.04.006
Ma JL, Yin BC, Wu X, Ye BC (2017) Simple and cost-effective glucose detection based on carbon nanodots supported on silver nanoparticles. Anal Chem 89(2):1323–1328. https://doi.org/10.1021/acs.analchem.6b04259
Gul U, Kanwal S, Tabassum S, Gilani MA, Rahim A (2020) Microwave-assisted synthesis of carbon dots as reductant and stabilizer for silver nanoparticles with enhanced-peroxidase like activity for colorimetric determination of hydrogen peroxide and glucose. Microchim Acta 187(2):135. https://doi.org/10.1007/s00604-019-4098-x
Wu S, Kong XJ, Cen Y, Yuan J, Yu RQ, Chu X (2016) Fabrication of a LRET-based upconverting hybrid nanocomposite for turn-on sensing of H2O2 and glucose. Nanoscale 8(16):8939–8946. https://doi.org/10.1039/c6nr00470a
Walekar LS, Hu P, Liao F, Guo X, Long M (2017) Turn-on fluorometric and colorimetric probe for hydrogen peroxide based on the in-situ formation of silver ions from a composite made from N-doped carbon quantum dots and silver nanoparticles. Microchim Acta 185(1):31. https://doi.org/10.1007/s00604-017-2545-0
Kong RM, Yang A, Wang Q, Wang Y, Ma L, Qu F (2018) Uricase based fluorometric determination of uric acid based on the use of graphene quantum dot@silver core-shell nanocomposites. Microchim Acta 185(63). https://doi.org/10.1007/s00604-017-2614-4
Kasprzyk W, Swiergosz T, Bednarz S, Walas K, Bashmakova NV, Bogdal D (2018) Luminescence phenomena of carbon dots derived from citric acid and urea - a molecular insight. Nanoscale 10(29):13889–13894. https://doi.org/10.1039/c8nr03602k
Ge B, Lisdat F (2002) Superoxide sensor based on cytochrome c immobilized on mixed-thiol SAM with a new calibration method. Anal Chim Acta 454(1):53–64. https://doi.org/10.1016/S0003-2670(01)01545-8
Maeda H, Yamamoto K, Nomura Y, Kohno I, Hafsi L, Ueda N, Yoshida S, Fukuda M, Fukuyasu Y, Yamauchi Y, Itoh N (2005) A design of fluorescent probes for superoxide based on a nonredox mechanism. J Am Chem Soc 127(1):68–69. https://doi.org/10.1021/ja047018k
Hu JJ, Wong NK, Ye S, Chen X, Lu MY, Zhao AQ, Guo Y, Ma AC, Leung AY, Shen J, Yang D (2015) Fluorescent probe HKSOX-1 for imaging and detection of endogenous superoxide in live cells and in vivo. J Am Chem Soc 137(21):6837–6843. https://doi.org/10.1021/jacs.5b01881
Li J, Zhang B, Wang F, C-y L (2011) Silver/carbon-quantum-dot plasmonic luminescent nanoparticles. New J Chem 35(3):554. https://doi.org/10.1039/c0nj01027h
Xia J, Di J, Li H, Xu H, Li H, Guo S (2016) Ionic liquid-induced strategy for carbon quantum dots/BiOX (X=Br, Cl) hybrid nanosheets with superior visible light-driven photocatalysis. Appl Catal B 181:260–269. https://doi.org/10.1016/j.apcatb.2015.07.035
Liu L, Gao Z, Jiang B, Bai Y, Wang W, Yin Y (2018) Reversible assembly and dynamic plasmonic tuning of Ag nanoparticles enabled by limited ligand protection. Nano Lett 18(8):5312–5318. https://doi.org/10.1021/acs.nanolett.8b02325
Zhan Y, Geng T, Liu Y, Hu C, Zhang X, Lei B, Zhuang J, Wu X, Huang D, Xiao G, Zou B (2018) Near-ultraviolet to near-infrared fluorescent nitrogen-doped carbon dots with two-photon and piezochromic luminescence. ACS Appl Mater Interfaces 10(33):27920–27927. https://doi.org/10.1021/acsami.8b07498
Han J, Liu Z, Guo Y, Han G-C, Li W, Chen S, Zhang S (2017) Determination of superoxide anion radical by modified CdTe quantum dots. J Photochem Photobiol A 349:1–6. https://doi.org/10.1016/j.jphotochem.2017.08.049
Li Z, Xiao L (2017) Facile sonochemical synthesis of water-soluble gold nanodots as fluorescent probes for superoxide radical anion detection and cell imaging. Anal Methods 9(12):1920–1927. https://doi.org/10.1039/c6ay03294j
Li P, Liu L, Xiao H, Zhang W, Wang L, Tang B (2016) A new polymer nanoprobe based on chemiluminescence resonance energy transfer for ultrasensitive imaging of intrinsic superoxide anion in mice. J Am Chem Soc 138(9):2893–2896. https://doi.org/10.1021/jacs.5b11784
Song Y, Hao J, Hu D, Zeng M, Li P, Li H, Chen L, Tan H, Wang L (2017) Ratiometric fluorescent detection of superoxide anion with polystyrene@nanoscale coordination polymers. Sensors Actuators B Chem 238:938–944. https://doi.org/10.1016/j.snb.2016.04.181
Li L, Chen Y, Gibson SB (2013) Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal 25(1):50–65. https://doi.org/10.1016/j.cellsig.2012.09.020
Sachdev A, Matai I, Gopinath P (2015) Dual-functional carbon dots-silver@zinc oxide nanocomposite: in vitro evaluation of cellular uptake and induction of apoptosis. J Mater Chem B 3(7):1217–1229. https://doi.org/10.1039/c4tb02043j
Tang M, Teng P, Long Y, Wang X, Liang L, Shen D, Wang J, Zheng H (2018) Hollow carbon dots labeled with FITC or TRITC for use in fluorescent cellular imaging. Microchim Acta 185(4):223. https://doi.org/10.1007/s00604-018-2761-2
Shi X, Meng H, Sun Y, Qu L, Lin Y, Li Z, Du D (2019) Far-red to near-infrared carbon dots: preparation and applications in biotechnology. Small 15(48):1901507. https://doi.org/10.1002/smll.201901507
Funding
This work was financially supported by grants from the Key Research and Development Project of Shanxi Province (International Cooperation Project, No. 201803D421087) and College Science and Technology Innovation Project of Shanxi Education Department (No. 201802040).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1393 kb)
Rights and permissions
About this article
Cite this article
Liang, H., Liu, H., Tian, B. et al. Carbon quantum Dot@Silver nanocomposite–based fluorescent imaging of intracellular superoxide anion. Microchim Acta 187, 484 (2020). https://doi.org/10.1007/s00604-020-04359-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04359-8