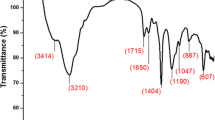
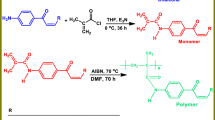
Abstract
The thermal decomposition behavior of polyacrylamide poly (methacryloyloxyethyl trimethylammonium chloride-acrylamide) (P(DMC-AM)) was studied by means of TG and DSC under nitrogen atmosphere at temperature range of 323.15–823.15 K. The effect of cationicity on thermal stability was discussed. The TG/DSC curves indicated that the decomposition process of P(DMC-AM) included three steps, and an increase in cationicity resulted in a slightly increased decomposition temperature and decomposition enthalpy, indicating a higher thermostability for P(DMC-AM). Furthermore, the evolved gases during the degradation were analyzed simultaneously via TG coupled with FTIR and MS. The possible thermal composition mechanism was proposed and validated by calculating the bond orders. The results from this study might supply useful message for expanding innovative application.









Similar content being viewed by others
References
Kitahara Y, Okuyama K, Ozawa K, Suga T, Takahashi S, Fujii T. Thermal decomposition of acrylamide from polyacrylamide. J Therm Anal Calorim. 2012;110(1):423–9. https://doi.org/10.1007/s10973-012-2544-7.
Feng L, Zheng H, Wang Y, Zhang S, Xu B. Ultrasonic-template technology inducing and regulating cationic microblocks in CPAM: characterization, mechanism and sludge flocculation performance. RSC Adv. 2017;7(38):23444–56. https://doi.org/10.1039/c7ra03784h.
Abdollahi Z, Frounchi M, Dadbin S. Synthesis, characterization and comparison of PAM, cationic PDMC and P(AM-co-DMC) based on solution polymerization. J Ind Eng Chem. 2011;17(3):580–6. https://doi.org/10.1016/j.jiec.2010.10.030.
Kesal D, Christau S, Trapp M, Krause P, von Klitzing R. The internal structure of PMETAC brush/gold nanoparticle composites: a neutron and X-ray reflectivity study. Phys Chem Chem Phys. 2017;19(45):30636–46. https://doi.org/10.1039/c7cp04404f.
Van Rossum R, Holappa S, Kyllonen L, inventors; Kemira Oyj, Finland. assignee. Method for preparing a dry cationic hydrogel polymer product, polymer product and its use. World pantent: WO2016079383A1. 2016.
Mahara S, Wang X, Dobashi Y, Sasadaira M, inventors; Sekisui Chemical Co., Ltd., Japan. assignee. Method of manufacturing a connection structure with increased viscosity of the binder resin in the conductive film to decrease the connection resistance between electrodes. World pantent: WO2017051872A1. 2017.
Qian J. Several questions about cationic polymer flocculants. Funct Polym. 2002;21:3–6.
Wang Q, Zhang W, Yang Z, Xu Q, Yang P, Wang D. Enhancement of anaerobic digestion sludge dewatering performance using in situ crystallization in combination with cationic organic polymers flocculation. Water Res. 2018;146:19–29. https://doi.org/10.1016/j.watres.2018.09.015.
De Buruaga AS, De la Cal JC, Asua JM. Inverse microemulsion polymerization of MADQUAT initiated with sodium metabisulfite. Polymer. 1999;41(4):1269–76.
He Y, Li G, Yang F, Yu X, Cui Y, Ren F. Precipitation polymerization of acrylamide with quaternary ammonium cationic monomer in potassium carbonate solution initiated by plasma. J Appl Polym Sci. 2007;104(6):4060–7. https://doi.org/10.1002/app.25649.
Goel NK, Rao MS, Kumar V, Bhardwaj YK, Chaudhari CV, Dubey KA, et al. Synthesis of antibacterial cotton fabric by radiation-induced grafting of [2-(methacryloyloxy)ethyl]trimethylammonium chloride (MAETC) onto cotton. Radiat Phys Chem. 2009;78(6):399–406. https://doi.org/10.1016/j.radphyschem.2009.03.011.
Ma Y, Sun X. Study of gelatin-AM-DMC graft copolymerization under microwave radiation and its strengthening effect to wood pulp. Adv Mater Res. 2011;233:1718–21. https://doi.org/10.4028/www.scientific.net/AMR.233-235.1718.
Zhang B, Lv X, An Z. Modular monomers with tunable solubility: synthesis of highly incompatible block copolymer nano-objects via RAFT aqueous dispersion polymerization. ACS Macro Lett. 2017;6(3):224–8. https://doi.org/10.1021/acsmacrolett.7b00056.
Zhang N, Wu Y, Xu J, Liu Y. Characterization and solution properties of acrylamide/(2-methacryloyloxyethyl) trimethylammonium chloride/dimethydiallylammonium chloride terpolymer. Chem Eng Commun. 2010;38(4):65–8.
Xu L, Che L, Zheng J, Huang G, Wu X, Chen P, et al. Synthesis and thermal degradation property study of N-vinylpyrrolidone and acrylamide copolymer. RSC Adv. 2014;4(63):33269–78. https://doi.org/10.1039/C4RA05720A.
Reber AC, Khanna SN, Ottenbrite R. Thermodynamic stability of polyacrylamide and poly(N, N-dimethyl acrylamide). Polym Adv Technol. 2007;18(12):978–85. https://doi.org/10.1002/pat.949.
Caulfield MJ, Qiao GG, Solomon DH. Some aspects of the properties and degradation of polyacrylamides. Chem Rev (Washington, DC, U S). 2002;102(9):3067–83. https://doi.org/10.1021/cr010439p.
Phang Y-N, Chee S-Y, Lee C-O, Teh Y-L. Thermal and microbial degradation of alginate-based superabsorbent polymer. Polym Degrad Stab. 2011;96(9):1653–61. https://doi.org/10.1016/j.polymdegradstab.2011.06.010.
Liu Y, Wang J, Zhu P, Zhao J, Zhang C, Guo Y, et al. Thermal degradation properties of biobased iron alginate film. J Anal Appl Pyrol. 2016;119:87–96. https://doi.org/10.1016/j.jaap.2016.03.014.
Trimpin S, Wijerathne K, McEwen CN. Rapid methods of polymer and polymer additives identification: multi-sample solvent-free MALDI, pyrolysis at atmospheric pressure, and atmospheric solids analysis probe mass spectrometry. Anal Chim Acta. 2009;654(1):20–5. https://doi.org/10.1016/j.aca.2009.06.050.
Tudorachi N, Chiriac AP. TGA/FTIR/MS study on thermal decomposition of poly(succinimide) and sodium poly(aspartate). Polym Test. 2011;30:397–407. https://doi.org/10.1016/j.polymertesting.2011.02.007.
Liu T, Sun C, Ma F. Study on the synthesis and thermal degradation of vinylphenylpolysilsesquioxane. J Anal Appl Pyrolysis. 2018;130:249–55. https://doi.org/10.1016/j.jaap.2017.12.024.
Zhang Y, Fu X. Preparation of P(DMC-AM) with high molecular weight and serial cationicities. Chinese patent: 201910073957.1. 2019.
de Carvalho GC, de Moura MDFV, de Castro HGC, da Silva JJH, da Silva HEB, dos Santos KM, et al. Influence of the atmosphere on the decomposition of vegetable oils: study of the profiles of FTIR spectra and evolution of gaseous products. J Therm Anal Calorim. 2020;140(5):2247–58. https://doi.org/10.1007/s10973-019-08960-9.
Feng L, Liu S, Zheng H, Liang J, Sun Y, Zhang S, et al. Using ultrasonic (US)-initiated template copolymerization for preparation of an enhanced cationic polyacrylamide (CPAM) and its application in sludge dewatering. Ultrason Sonochem. 2018;44:53–63. https://doi.org/10.1016/j.ultsonch.2018.02.017.
Ma J, He L, Huang L, Peng Q, Cai X. Synthesis of aliphatic–aromatic polyamide carbonized system with phosphoramide structure and study on its thermal degradation mechanism and flame retardancy in polypropylene system. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09879-2.
Tang L, Zhang Y. Research on the preparation of poly-methacrylatoethyltrimethyl ammonium chloride(PDMC). Fine Chem. 2014;31(11):1324–9.
Li X, Liu B, Zhao C, Zheng H, Gao B, Sun Y. UV-initiated template copolymerization of AM and MAPTAC: microblock structure, copolymerization mechanism, and flocculation performance. Chemosphere. 2017;167:71–81.
Tutas M, Saglam M, Yuksel M. Investigation of pyrolysis products of polyacrylamide by pyrolysis-gas chromatography. J Anal Appl Pyrolysis. 1991;22(1–2):129–37. https://doi.org/10.1016/0165-2370(91)85012-V.
Zan L-N, Nie L-H, Yang P, Yuan Q. The synthesis of poly(DAC-AM). Huaxue Tuijinji Yu Gaofenzi Cailiao. 2009;7(1):34–5.
Caglar B, Coldur F, Caglar S, Cubuk O, Tabak A, Topcu C. Structural, thermal and morphological properties of a novel poly(acrylamide-co-methacrylic acid)/organoclay nanocomposite. Inorg Nano-Met Chem. 2017;47(3):360–4. https://doi.org/10.1080/15533174.2016.1186063.
Singh A, Singh S, Sharma TC, Kishore P. Physicochemical properties and kinetic analysis for some fluoropolymers by differential scanning calorimetry. Polym Bull (Heidelberg, Ger). 2017;75(6):2315–38. https://doi.org/10.1007/s00289-017-2153-5.
Dmitrieva TV, Sirovatka LA, Bortnitskii VI. Effect of preliminary mechanical activation of polyacrylamide in the presence of metal on polymer thermal decomposition. Zh Prikl Khim (S-Peterburg). 1998;71(10):1709–12.
Mallick L, Kumar S, Chowdhury A. Thermal decomposition of ammonium perchlorate-A TGA-FTIR-MS study: part II. Thermochim Acta. 2017;610:57–68. https://doi.org/10.1016/j.tca.2017.04.004.
Hamciuc C, Lisa G, Hamciuc E, Tudorachi N. Thermal decomposition study of some polyimide-polydimethylsiloxane copolymers. J Anal Appl Pyrolysis. 2018;129:204–14. https://doi.org/10.1016/j.jaap.2017.11.011.
Gu X, Ma X, Li L, Liu C, Cheng K, Li Z. Pyrolysis of poplar wood sawdust by TG-FTIR and Py–GC/MS. J Anal Appl Pyrol. 2013;102:16–23. https://doi.org/10.1016/j.jaap.2013.04.009.
Li Y-Y, Ren N, He S-M, Zhang J-J. Supramolecular structures, thermal decomposition mechanism and heat capacity of the novel binuclear Tb(III) and Dy(III) complexes with 2,3-dimethoxybenzoic acid and 5,5′-dimety-2,2′-bipyridine. J Therm Anal Calorim. 2020;140(5):2435–45. https://doi.org/10.1007/s10973-019-08944-9.
Mohsin IU, Lager D, Gierl C, Hohenauer W, Danninger H. Thermo-kinetics study of MIM thermal de-binding using TGA coupled with FTIR and mass spectrometry. Thermochim Acta. 2010;503–504:40–5. https://doi.org/10.1016/j.tca.2010.03.005.
Tomescu M, Cretu S, Chivulescu E, Ciohodaru L, Dragutescu M. Thermal degradation of acrylamide-maleic anhydride copolymer. Mater Plast (Bucharest). 1985;22(2):108–10.
Coleman MM, Gordon B III. The degradation of acrylonitrile—acrylamide copolymers. Anal Proc (London). 1983;20(11):572–4.
King B, Lessard BH. Controlled synthesis and degradation of poly(N-(isobutoxymethyl) acrylamide) homopolymers and block copolymers. Macromol React Eng. 2017. https://doi.org/10.1002/mren.201600073.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, X., Yang, Q. & Zhang, Y. Thermal decomposition behavior and mechanism study of cationic polyacrylamide. J Therm Anal Calorim 146, 1371–1381 (2021). https://doi.org/10.1007/s10973-020-10131-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10131-0