Abstract
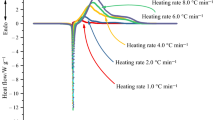
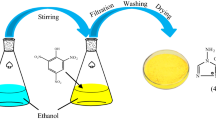
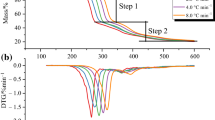
Owing to splendid properties, trinitrophenol-based ionic liquid consisting of ammonium–imidazolium cation, 3,5-diamino-1,2,4-triazole picrate (DATPA), has been investigated concerning the thermal stability in this study. Differential scanning calorimeter (DSC) and thermogravimetry (TG) are used to explore the thermal behavior such as melting point, decomposition temperature, and the temperature of 10% mass loss which are the target to evaluate the thermal stability of other two picrates (picrate and 4-amino-1,2,4-triazole picrate). The results show that DATPA has better thermal stability at 251–252 °C than the other two picrates, and the melt volatility has decreased as well. Moreover, thermal kinetics parameters are calculated by changing the heating rates of DSC and TG. ASTM standard E698 and Kissinger methods are utilized to calculate, and double extrapolation is accepted to explore the mechanism function in the decomposition course, which has been defined as \(f(\alpha ) = 2.105(1{-}\alpha )[\ln (1{-}\alpha )]^{0.525}\) in this study. Finally, an accelerating rate calorimeter is employed to study the thermal hazard of DATPA under the adiabatic condition and evaluate the level of runaway reaction through the risk matrix. According to Townsend method, we achieve the thermal kinetics parameters of DATPA under adiabatic condition. The results show that the apparent activation energy (Ea) of DATPA is 273.2 kJ mol−1 higher than many other explosives. Besides, according to the theoretical calculation, we find that DATPA has good detonation properties with high stability.










Similar content being viewed by others
References
Plechkova NV, Seddon KR. Applications of ionic liquids in the chemical industry. Chem Soc Rev. 2008;37:123–50.
Javed F, Ullah F, Zakaria MR, Akil HM. An approach to classification and hi-tech applications of room-temperature ionic liquids (RTILs): a review. J Mol Liq. 2018;271:403–20.
Mei X, Yue Z, Ma Q, Dunya H, Mandal BK. Synthesis and electrochemical properties of new dicationic ionic liquids. J Mol Liq. 2018;272:1001–18.
Sengupta D, Vaghjiani GL. Molecular orbital based design guideline for hypergolic ionic liquids. Propellants Explos Pyrotech. 2015;40:144–9.
Pagoria PF, Lee GS, Mitchell AR, Schmidt RD. A review of energetic materials synthesis. Thermochim Acta. 2002;384:187–204.
Singh RP, Verma RD, Meshri DT, Shreeve JM. Energetic nitrogen-rich salts and ionic liquids. Angew Chem Int Ed. 2006;45:3584–601.
Soriano-García M, Schatz-Levine M, Toscano RA, Villena Iribe R. Structure of the complex of imidazole and picric acid. Acta Crystallogr Sect C Cryst Struct Commun. 1990;46:1556–8.
Matsukawa M, Matsunaga T, Yoshida M, Fujiwara S. Synthesis and properties of lead picrates. Sci Technol Energy Mater. 2004;65:7–13.
Wu Q, Zhu W, Xiao H. A new design strategy for high-energy low-sensitivity explosives: combining oxygen balance equal to zero, a combination of nitro and amino groups, and N-oxide in one molecule of 1-amino-5-nitrotetrazole-3N-oxide. J Mater Chem A. 2014;2:13006–15.
Ogihara W, Yoshizawa M, Ohno H. Novel ionic liquids composed of only azole ions. Chem Lett. 2004;33:1022–3.
Jaidann M, Roy S, Abou-Rachid H, Lussier LS. A DFT theoretical study of heats of formation and detonation properties of nitrogen-rich explosives. J Hazard Mater. 2010;176:165–73.
Yu YW. Theoretical calculation of the detonation parameters of 2,6-diamino-3,5-dinitropyrazine-1-oxide. Initiat Pyrotech. 2006;2:31.
Ren Y, Zhang C, Zhang X, Zhao F, Ma H, Xu K. Specific heat capacity, thermodynamic properties and characterization of detonation velocity and pressure of picrate of 3, 5-diamino-1, 2, 4-triazole. J Northwest Univ Nat Sci Ed. 2012;13:56–9.
Agrawal JP, Field JE. Recent trends in high-energy materials. Prog Energy Combust Sci. 1998;24:1–30.
Elbeih A, Abd-Elghany M, Klapötke TM. Kinetic parameters of PBX based on Cis-1, 3, 4, 6-tetranitroocta-hydroimidazo-[4, 5-d] imidazole obtained by isoconversional methods using different thermal analysis techniques. Propellants Explos Pyrotech. 2017;42:468–76.
Blaine RL, Kissinger HE. Homer Kissinger and the Kissinger equation. Thermochim Acta. 2012;540:1–6.
Xiang P, Ying GX, Yuan FZ. Non isothermal kinetics of the dehydration process of cobalt (II) oxalate dihydrate in solid state using double extrapolation. J Chem Res Chin Univ. 1999;7:1449–53.
Jiang HC, Lin WC, Hua M, Pan XH, Shu CM, Jiang JC. Analysis of kinetics of thermal decomposition of melamine blended with phosphorous ionic liquid by green approach. J Therm Anal Calorim. 2017;131:2821–31.
Pasturenzi C, Dellavedova M, Gigante L, Lunghi A, Canavese M, Cattaneo CS. Thermochemical stability: a comparison between experimental and predicted data. J Loss Prev Process Ind. 2014;28:79–91.
Zhang Y, Ni L, Jiang J, Jiang J, Zhang W, Jiang J. Thermal hazard analyses for the synthesis of benzoyl peroxide. J Loss Prev Process Ind. 2016;43:35–41.
Zhang L, Yu WD, Pan XH, Fang JJ, Hua M, Chen FM. Thermal hazard assessment for synthesis of 3-methylpyridine-N-oxide. J Loss Prev Process Ind. 2015;35:316–20.
Liu H, Gu L, Zhu P, Liu Z, Zhou B. Evaluation on thermal hazard of ter-butyl hydroperoxide by using accelerating rate calorimeter. Procedia Eng. 2012;45:574–9.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimeter. Thermochim Acta. 1980;37:1–30.
Lu GB, Zhang CX, Chen WH, Chen LP, Zhou YS. Thermal hazards and kinetic analysis of salicyl hydroxamic acid under isothermal and adiabatic conditions. Thermochim Acta. 2016;623:43–9.
Acknowledgements
The authors would like to express their appreciation to the Anhui Provincial Natural Science Foundation, China, for its financial support of this study under the Contract Number 1908085ME125.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, SH., Li, JJ., Zhang, B. et al. Study of thermal behavior and hazard of DATPA based on comprehensive thermodynamic analysis. J Therm Anal Calorim 144, 315–324 (2021). https://doi.org/10.1007/s10973-020-10124-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10124-z