Remediation of Anthracene-Contaminated Soil with Sophorolipids-SDBS-Na2SiO3 and Treatment of Eluting Wastewater
Abstract
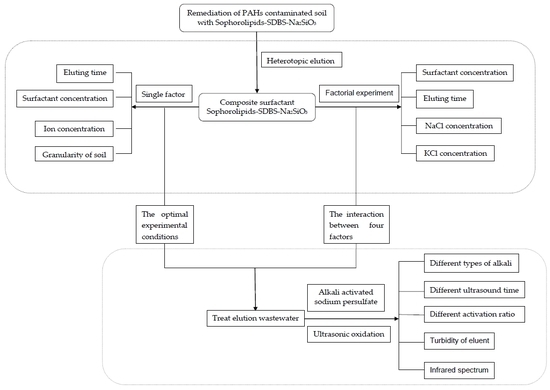
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soil Washing Using Mixed Surfactants
2.3. Eluant Using Alkali-Activated Sodium Persulfate
2.4. Removal Effectiveness Evaluation
2.5. Analytical Methods
2.6. Data Analysis
3. Results and Discussion
3.1. Experiment of Single Surfactant Remediation of Anthracene-Contaminated Soil
3.2. Effect of Mixed Surfactants on the Single Factor of Anthracene-Contaminated Soil Eluting
3.2.1. Effect of Eluting Time
3.2.2. Mixed Surfactant Concentration
3.2.3. Effect of Ion Concentration
3.2.4. Effect of Granularity
3.3. Effect of Mixed Surfactants on the Eluting Interaction of PAH-Contaminated Soils
3.3.1. Design Scheme of Mixed Surfactant on Eluting PAH-Contaminated Soil
3.3.2. Main Effect Analysis
3.3.3. Interaction Analysis
3.4. Removal of Anthracene from Eluent by the Base/SPS
3.4.1. The Oxidation Effects of Different Types of Alkali Activation on the Eluent
3.4.2. Effect of Different Ultrasound Times on the Base of Sodium Persulfate
3.4.3. Effect of Different Activation Ratios on the Base of Sodium Persulfate
3.4.4. Effect of Turbidity of Eluent
3.4.5. Results and Analysis of Elution Waste Liquid by Infrared Spectrum
4. Conclusions
- (1)
- In the single factor experiment, the best concentration of sophora and SDBS was 40 and 100 mg/L, respectively, that is to say, the removal efficiency was best at the critical micelle concentration. At the same time, the additive sodium silicate can react with the acidic components of oil to form salt, which increases the water solubility of oil and improves the removal efficiency of the composite surfactant.
- (2)
- When the elution time was 8 h, the concentration of the compounded surfactant was 1200 mg/L, the particle size was 60 mesh, the concentration of NaCl was 100 mmol/L, and the concentration of KCl was 50 mmol/L, thus the effect of the PAH-contaminated soil eluted by the composite surfactant was the best; externally added NaCl and KCl salt ions had a more obvious promotion effect on the polycyclic aromatic hydrocarbon-contaminated soil.
- (3)
- In the factorial design experiment, the residual plot of the regression equation conformed to the normal distribution, and the p value of the error analysis was less than 0.0018, which showed that the regression equation simulation was significant. It can be seen from the Pareto analysis chart that single factor B (elution time) and D (NaCl concentration) have a significant main effect. There is also a certain interaction between factor A (concentration agent concentration) and factor D, factor B, and factor C (KCl concentration).
- (4)
- The ultrasonic oxidation technology was used, combined with alkali-activated sodium persulfate, to explore the influence of the ultrasonic time, activation ratio, turbidity, and other factors on the oxidation effect. When the optimal ultrasonic time was 60 min and the ratio of oxidant to activator was 1:2, the removal rate of contaminants in the eluent could reach 63.7%. At the same time, the turbidity of the eluent was significantly lower than that of the liquid after centrifugal separation, indicating that oxidants can not only remove the pollutants in elution water but also remove the residual soil particulate matter.
- (5)
- By comparing the infrared spectrum of the eluted waste liquid before and after oxidation, it was seen that during the oxidation process, the inner part of eluent waste liquid underwent a ring-opening reaction, and the ring-opening reaction also occurred in the part of the cyclic ester group of the surfactant, which changed from a ring to non-ring.
Author Contributions
Funding
Conflicts of Interest
References
- Shi, Z.; Chen, J.; Liu, J.; Wang, N.; Sun, Z.; Wang, X. Anionic-nonionic Mixed-surfactants-enhanced remediation of PAH-contaminated soil. Environ. Sci. Pollut. Res. Int. 2015, 22, 12769–12774. [Google Scholar] [CrossRef] [PubMed]
- Lane, W.F.; Loehr, R.C. Estimating the equilibrium aqueous concentrations of polynuclear aromatic hydrocarbons in complex mixtures. Water Environ. Res. 1995, 67, 169–173. [Google Scholar] [CrossRef]
- Paria, S.; Yuet, P.K. Solubilization of Naphthalene by Pure and Mixed Surfactants. Ind. Eng. Chem. Res. 2006, 45, 3552–3558. [Google Scholar] [CrossRef]
- Lamichhane, S.; Bal Krishna, K.C.; Sarukkalige, R. Surfactant–enhanced remediation of polycyclic aromatic hydrocarbons: A review. J. Environ. Manag. 2017, 199, 46–61. [Google Scholar] [CrossRef]
- Zhai, M.; Huang, G.; Liu, L.; Zheng, B.; Guan, Y. Inter-regional carbon flows embodied in electricity transmission: Network simulation for energy-carbon nexus. Renew. Sustain. Energy Rev. 2020, 118, 109511. [Google Scholar] [CrossRef]
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X.; Berenguer, M.J. VOCs and PAHs emissions from creosote-treated wood in a field storage area. Sci. Total Environ. 2008, 402, 130–138. [Google Scholar] [CrossRef]
- Ravindra, K.; Sokhi, R.; René, V.G. Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 2008, 42, 2895–2921. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.H.; Ong, S.K.; Golchin, J.; Nelson, G.S. Use of solvents to enhance PAH biodegradation of coal tar-contaminated soils. Water Res. 2001, 35, 3941–3949. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, L.; Zhou, W. Enhanced desorption and biodegradation of phenanthrene in soil–water systems with the presence of anionic–nonionic mixed surfactants. J. Hazard. Mater. 2007, 142, 354–361. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, L. Efficiency of surfactant-enhanced desorption for contaminated soils depending on the component characteristics of soil-surfactant–PAHs system. Environ. Pollut. 2007, 147, 73. [Google Scholar] [CrossRef]
- Xu, H.; Ho, S.S.H.; Gao, M.; Cao, J.; Guinot, B.; Ho, K.F.; Long, X.; Wang, J.; Shena, Z.; Liu, S.; et al. Microscale spatial distribution and health assessment of PM2.5-bound polycyclic aromatic hydrocarbons (PAHs) at nine communities in Xi’an, China. Environ. Pollut. 2016, 218, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.H.; Jing, D.D.; Wan, J.; Ma, Y.; Wang, Y. Temporal and spatial variations of contaminant removal, enzyme activities, and microbial community structure in a pilot horizontal subsurface flow constructed wetland purifying industrial run off. Environ. Sci. Pollut. Res. 2016, 23, 8565–8576. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Huang, G.; Liu, H.; Liu, L.; He, C.; Liu, Z. Three-perspective energy-carbon nexus analysis for developing China’s policies of CO2-emission mitigation. Sci. Total Environ. 2020, 705, 135857. [Google Scholar] [CrossRef]
- Ji, H.J.; Long, T.; Chen, Q.; He, Y.; Lin, Y.S.; Yu, R.; Zhu, X. Tween 80 enhanced activated sodium persulfate oxidation remediation of PAHs contaminated soil. Chem. Eng. J. 2016, 67, 3879–3887. [Google Scholar]
- Wang, H.T.; Zhu, H.; Wei, X.; Liang, Y.; Lu, X.Y. The effect of sodium humate and surfactant on desorption and solubilization of petroleum pollutants in loess. J. Saf. Environ. 2004, 4, 2–55. [Google Scholar]
- Yang, K.; Zhu, L.; Xing, B. Enhanced soil washing of phenanthrene by mixed solutions of TX100 and SDBS. Environ. Sci. Technol. 2006, 40, 4274–4280. [Google Scholar] [CrossRef]
- Wang, Z.R.; Sun, F.L.; Pu, C.X. The leaching treatment of polycyclic aromatic hydrocarbon contaminated soil by mixed surfactants. China Water Supply Drain. 2019, 35, 92–96. [Google Scholar]
- Zhang, L.; Somasundaran, P.; Singh, S.K.; Felse, A.P.; Gross, R. Synthesis and interfacial properties of sophorolipid derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2004, 240, 75–82. [Google Scholar] [CrossRef]
- Pekin, G.; Vardar-Sukan, F.; Kosaric, N. Production of Sophorolipids from Candida bombicola ATCC 22214 Using Turkish Corn Oil and Honey. Eng. Life Sci. 2005, 5, 357–362. [Google Scholar] [CrossRef]
- Hirata, Y.; Ryu, M.; Oda, Y.; Igarashi, K.; Nagatsuka, A.; Furuta, T.; Sugiura, M. Novel characteristics of sophorolipids, yeast glycolipid biosurfactants, as biodegradable low-foaming surfactants. J. Biosci. Bioeng. 2009, 108, 142–146. [Google Scholar] [CrossRef]
- Sun, Y.X.; Yang, Z.L.; Yu, G.H.; Zhan, R.; Liang, S.K. Study on the bioremediation of oil-containing sludge with sophorolipids surfactant and petroleum hydrocarbon degrading bacteria. J. Ocean. Univ. China 2017, 47, 72–80. [Google Scholar]
- Li, Z.S.; Liang, S.K.; Li, J.F. Experimental study on electrokinetic remediation of chromium contaminated soil with sophorolipids biosurface. J. Ocean. Univ. China 2019, 49, 33–43. [Google Scholar]
- Deshpande, S.; Shiau, B.J.; Wade, D.; Sabatini, D.A.; Harwell, J.H. Surfactant selection for enhancing ex situ soil washing. Water Res. 1999, 33, 351–360. [Google Scholar] [CrossRef]
- Fabbri, D.; Prevot, A.B.; Zelano, V.; Ginepro, M.; Pramauro, E. Removal and degradation of aromatic compounds from a highly polluted site by coupling soil washing with photocatalysis. Chemosphere 2008, 71, 59–65. [Google Scholar] [CrossRef]
- Gao, S.X.; Cao, J.S.; Sun, C.; Wang, L.S. Solubilization of 1, 2, 4-trichlorobenzene by different surfactants. Soil Environ. 1999, 184–188. [Google Scholar]
- Ni, H.W. Anionic-Non-Ionic Mixed Surfactants Strengthen Plant-Microorganism Joint Remediation of Pahs Contaminated Soil. Master’s Thesis, Zhejiang University, Hangzhou, China, 2014. [Google Scholar]
- He, Z.N.; Li, Z.S.; Ji, G.D. Simulation study on oil recovery from oil field contaminated soil. J. Appl. Found. Eng. Sci. 2005, 2, 136–145. [Google Scholar]
- Lee, D.H.; Chang, H.W.; Kim, C. Mixing effect of NaCl and surfactant on the remediation of TCB contaminated soil. Geoences J. 2008, 12, 63–68. [Google Scholar] [CrossRef]
- Tsomides, H.J. Effect of surfactant addition on phenanthrene biodegradation in sediments. Environ. Toxicol. Chem. 1994, 6, 953–959. [Google Scholar] [CrossRef]
- Kim, I.S.; Park, J.S.; Kim, K.W. Enhanced Biodegradation of Polycyclic Aromatic Hydrocarbons Using Nonionic Surfactants in Soil Slurry. Appl. Geochem. 2001, 16, 1419–1428. [Google Scholar] [CrossRef]
- Devi, P.; Das, U.; Dalai, A.K. In-situ chemical oxidation: Principle and applications of peroxide and persulfate treatments in wastewater systems. Sci. Total Environ. 2016, 571, 643–657. [Google Scholar] [CrossRef]
- Lominchar, M.A.; Lorenzo, D.; Romero, A.; Santos, A. Remediation of soil contaminated by PAHs and TPH using alkaline activated persulfate enhanced by surfactant addition at flow conditions. J. Chem. Technol. Biotechnol. 2017, 93, 1270–1278. [Google Scholar] [CrossRef]
- Qi, C.; Liu, X.; Ma, J.; Lin, C.; Li, X.; Zhang, H. Activation of peroxymonosulfate by base: Implications for the degradation of organic pollutants. Chemosphere 2016, 151, 280–288. [Google Scholar] [CrossRef] [PubMed]
- San Roman, I.; Galdames, A.; Alonso, M.L.; Bartolome, L.; Vilas, J.L.; Alonso, R.M. Effect of coating on the environmental applications of zero valent iron nanoparticles: The lindane case. Sci. Total Environ. 2016, 565, 795–803. [Google Scholar] [CrossRef]
- Gilman, D.V.; Ovanes, G.M.; Gerald, T.A.; Daniel, J.C. A QSAR analysis of substituent effects on the photoinduced acute toxicity of PAHs. Chemosphere 1995, 30, 2129–2142. [Google Scholar]
- Wu, W.; Chen, J.J.; Peng, S. Effect of process parameters on surfactant remediation of PAHs contaminated soil. J. Environ. Eng. 2012, 6, 977–982. [Google Scholar]
- Furman, O.S.; Teel, A.L.; Watts, R.J. Mechanism of Base Activation of Persulfate. Environ. Sci. Technol. 2010, 44, 6423–6428. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.D.; Zhou, G.H. Heterotopic chemical leaching to repair petroleum contaminated soil. J. Peking Univ. 2007, 43, 863–871. [Google Scholar]
- Kile, D.E.; Chiou, C.T. Water solubility enhancements of DDT and trichlorobenzene by some surfactants below and above the critical micelle concentration. Environ. Sci. Technol. 1989, 23, 832–838. [Google Scholar] [CrossRef]
- Lee, D.H.; Chang, H.W.; Cody, R.D. Synergism effect of mixed surfactant solutions in remediation of soil contaminated with PCE. Geoences J. 2004, 8, 319–323. [Google Scholar] [CrossRef]
- Jiang, B. Study on the Solubilization and Elution Mechanism of Mixed Surfactants for Pahs. Master’s Thesis, Lanzhou Jiaotong University, Lanzhou, China, 2007. [Google Scholar]
- Li, S. The Washing Remediation of the Contaminated Soil by Pahs with Surfactants. Master’s Thesis, Shenyang University, Shenyang, China, 2017. [Google Scholar]
- Ran, D.Q. Heterotopic Leaching Method to Repair Oil Contaminated Soil. Master’s Thesis, Shandong University, Jinan, China, 2012. [Google Scholar]
- Chen, G. Remediation of the Oil Contaminated Soil by Surfactants. Master’s Thesis, Shangdong University of Science and Technology, Qingdao, China, 2008. [Google Scholar]
- Sen, M.U. Experimental Study on Removal of Phenanthrene from Water by Gemini Surfactant Modified Sepiolite. Master’s Thesis, North China Electric Power University, Beijing, China, 2016. [Google Scholar]
- Ma, H. Study on Remediation of Petroleum Hydrocarbon Contaminated Soil by CMC Solubilization Combined with SPS Chemical Oxidation. Master’s Thesis, Chongqing University, Chongqing, China, 2016. [Google Scholar]
- Liang, C.; Guo, Y.Y. Remediation of Diesel-Contaminated Soils Using Persulfate Under Alkaline Condition. Water Air Soil Pollut. 2012, 223, 4605–4614. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, X.; Yan, X.; Huling, S.G.; Chai, T.; Tao, H. Effect and mechanism of persulfate activated by different methods for PAHs removal in soil. J. Hazard. Mater. 2013, 254, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.P. Study and Application of Surfactant Ectopic Washing Oil Contaminated Soil. Master’s Thesis, Dong Hua University, Shanghai, China, 2015. [Google Scholar]















| Surfactant | Purity | Molecular Formula | CMC (mg/L) |
|---|---|---|---|
| Sophorolipids | AR | C34H56O14 | 40 |
| SDBS | AR | C18H29NaO3S | 100 |
| Na2SiO3 | AR | Na2SiO3 | - |
| pH (H2O) | Organic Matter Content (%) | Moisture Content (%) | Cation Exchange Capacity (cmol/kg) | Salinity (%) | Particle Diameter (mm) |
|---|---|---|---|---|---|
| 8.59 | 0.75 | 2.63 | 7.73 | 0.55 | 2 |
| Run | Mixed-Surfactant Concentration/mg·L−1 | Eluting Time/h | KCl/mol·L−1 | NaCl/mol·L−1 | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | |||||
| Coded | Actual | Coded | Actual | Coded | Actual | Coded | Actual | |
| 1 | 1 | 1500 | 1 | 12 | 1 | 0.05 | 1 | 0.1 |
| 2 | 1 | 1500 | −1 | 6 | 1 | 0.05 | 1 | 0.1 |
| 3 | 1 | 1500 | −1 | 6 | −1 | 0 | −1 | 0 |
| 4 | 1 | 1500 | 1 | 12 | 1 | 0.05 | −1 | 0 |
| 5 | 1 | 1500 | −1 | 6 | −1 | 0 | 1 | 0.1 |
| 6 | −1 | 500 | 1 | 12 | 1 | 0.05 | −1 | 0 |
| 7 | −1 | 500 | −1 | 6 | 1 | 0.05 | −1 | 0 |
| 8 | −1 | 500 | 1 | 12 | 1 | 0.05 | 1 | 0.1 |
| 9 | −1 | 500 | 1 | 12 | −1 | 0 | 1 | 0.1 |
| 10 | −1 | 500 | −1 | 6 | −1 | 0 | 1 | 0.1 |
| 11 | −1 | 500 | 1 | 12 | −1 | 0 | −1 | 0 |
| 12 | 1 | 1500 | 1 | 12 | −1 | 0 | 1 | 0.1 |
| 13 | 1 | 1500 | −1 | 6 | 1 | 0.05 | −1 | 0 |
| 14 | 1 | 1500 | 1 | 12 | −1 | 0 | −1 | 0 |
| 15 | −1 | 500 | −1 | 6 | −1 | 0 | −1 | 0 |
| 16 | −1 | 500 | −1 | 6 | 1 | 0.05 | 1 | 0.1 |
| Source | Sum of Square | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| model | 0.167 | 12 | 0.014 | 86.77 | 0.0018 |
| A | 0.002 | 1 | 0.002 | 12.34 | 0.0391 |
| B | 0.012 | 1 | 0.012 | 75.67 | 0.0032 |
| D | 0.013 | 1 | 0.013 | 83.58 | 0.0028 |
| AB | 0.003 | 1 | 0.003 | 21.13 | 0.0194 |
| AC | 0.032 | 1 | 0.032 | 200.37 | 0.0008 |
| AD | 0.001 | 1 | 0.001 | 5.77 | 0.0957 |
| BC | 0.010 | 1 | 0.010 | 60.90 | 0.0044 |
| BD | 0.052 | 1 | 0.052 | 324.18 | 0.0004 |
| CD | 0.008 | 1 | 0.008 | 51.62 | 0.0056 |
| ABC | 0.003 | 1 | 0.003 | 21.72 | 0.0186 |
| ABD | 0.005 | 1 | 0.005 | 30.97 | 0.0114 |
| ABCD | 0.025 | 1 | 0.025 | 152.99 | 0.0011 |
| Cor Total | 0.167 | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, X.; Shi, L.; Du, X.; Wang, Z. Remediation of Anthracene-Contaminated Soil with Sophorolipids-SDBS-Na2SiO3 and Treatment of Eluting Wastewater. Water 2020, 12, 2188. https://doi.org/10.3390/w12082188
Li W, Wang X, Shi L, Du X, Wang Z. Remediation of Anthracene-Contaminated Soil with Sophorolipids-SDBS-Na2SiO3 and Treatment of Eluting Wastewater. Water. 2020; 12(8):2188. https://doi.org/10.3390/w12082188
Chicago/Turabian StyleLi, Wei, Xiaofeng Wang, Lixiang Shi, Xianyuan Du, and Zhansheng Wang. 2020. "Remediation of Anthracene-Contaminated Soil with Sophorolipids-SDBS-Na2SiO3 and Treatment of Eluting Wastewater" Water 12, no. 8: 2188. https://doi.org/10.3390/w12082188