Preserving Biodiversity in Marginal Rural Areas: Assessment of Morphological and Genetic Variability of a Sicilian Common Bean Germplasm Collection
Abstract
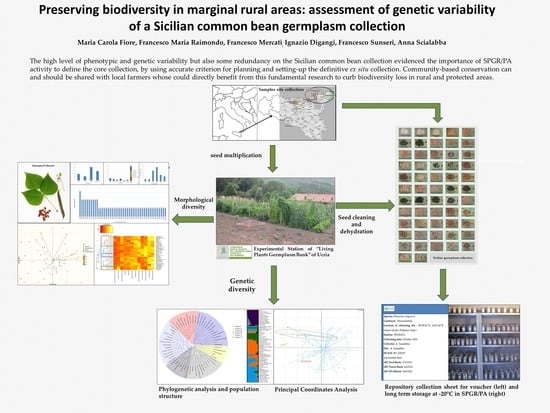
:1. Introduction
2. Results
2.1. Morphological Diversity
2.2. Genetic Diversity
3. Discussion and Conclusions
4. Materials and Methods
4.1. Plant Material
4.2. Morpho-Phenotypic Seed Analysis
4.3. DNA Isolation and Amplification
4.4. Data Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beebe, S.; Gonzalez, A.V.; Rengifo, J. Research on trace minerals in the common bean. Food Nutr. Bull. 2000, 21, 387–391. [Google Scholar] [CrossRef]
- Gepts, P.; Debouck, D. Origin, domestication, and evolution of the common bean (Phaseolus vulgaris L.). In Common Beans: Research for Crop Improvement; van Schoonhoven, A., Voysest, O., Eds.; C.A.B. Int.: Wallingford, UK; CIAT: Cali, Colombia, 1991; pp. 7–53. [Google Scholar]
- Gepts, P.; Bliss, F.A. Phaseolin variability among wild and cultivated common beans (Phaseolus vulgaris) from Colombia. Econ. Bot. 1986, 40, 469–478. [Google Scholar] [CrossRef]
- Gepts, P.; Bliss, F.A. Dissemination pathways of common bean (Phaseolus vulgaris, Fabaceae) deduced from phaseolin electrophoretic variability. II. Europe and Africa. Econ. Bot. 1988, 42, 86–104. [Google Scholar] [CrossRef]
- Singh, S.P.; Nodari, R.; Gepts, P. Genetic diversity in cultivated common bean: I. allozymes. Crop. Sci. 1991, 31, 19–23. [Google Scholar] [CrossRef]
- Debouck, D.G.; Toro, O.; Paredes, O.M.; Johnson, W.C.; Gepts, P. Genetic diversity and ecological distribution of Phaseolus vulgaris (Fabaceae) in northwestern South America. Econ. Bot. 1993, 47, 408–423. [Google Scholar] [CrossRef]
- Svetleva, D.; Pereira, G.; Carlier, J.; Cabrita, L.; Leitao, J.; Genchev, D. Molecular characterization of Phaseolus vulgaris L. genotypes included in Bulgarian collection by ISSR and AFLP™ analyses. Sci. Hortic. 2006, 109, 198–206. [Google Scholar] [CrossRef]
- Šustar-Vozlič, J.; Maras, M.; Javornik, B.; Meglič, V. Genetic diversity and origin of slovene common bean (Phaseolus vulgaris L.) germplasm as revealed by AFLP markers and phaseolin analysis. J. Am. Soc. Hortic. Sci. 2006, 131, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Sicard, D.; Nanni, L.; Porfiri, O.; Bulfon, D.; Papa, R. Genetic diversity of Phaseolus vulgaris L. and P. coccineus L. landraces in central Italy. Plant Breed. 2005, 124, 464–472. [Google Scholar] [CrossRef]
- Desiderio, F.; Bitocchi, E.; Bellucci, E.; Rau, D.; Rodriguez, M.; Attene, G.; Papa, R.; Nanni, L. Chloroplast microsatellite diversity in Phaseolus vulgaris. Front. Plant Sci. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Blair, M.W.; Giraldo, M.C.; Buendía, H.F.; Tovar, E.; Duque, M.C.; Beebe, S. Microsatellite marker diversity in common bean (Phaseolus vulgaris L.). Appl Genet. 2006, 113, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.; Gepts, P. Structure of genetic diversity in the two major gene pools of common bean (Phaseolus vulgaris L., Fabaceae). Theor. Appl. Genet. 2009, 118, 979–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, M.W.; Díaz, J.M.; Hidalgo, R.; Diaz, L.M.; Duque, M.C. Microsatellite characterization of Andean races of common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2007, 116, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Papa, R.; Nanni, L.; Sicard, D.; Rau, D. The evolution of genetic diversity in Phaseolus vulgaris L. In Darwin’s Harvest: New Approaches to the Origins; Evolution and Conservation of Crops; Motley, T.J., Zerega, N., Cross, H., Eds.; Columbia University Press: New York, NY, USA, 2006; pp. 121–143. [Google Scholar] [CrossRef]
- Papa, R.; Bellucci, E.; Rossi, M.; Leonardi, S.; Rau, D.; Gepts, P.; Nanni, L.; Attene, G. Tagging the Signatures of Domestication in Common Bean (Phaseolus vulgaris) by Means of Pooled DNA Samples. Ann. Bot. 2007, 100, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Bitocchi, E.; Bellucci, E.; Giardini, A.; Rau, D.; Rodriguez, M.; Biagetti, E.; Santilocchi, R.; Spagnoletti Zeuli, P.; Gioia, T.; Logozzo, G.; et al. Molecular analysis of the parallel domestication of the common bean (Phaseolus vulgaris) in Mesoamerica and the Andes. New Phytol. 2013, 197, 300–313. [Google Scholar] [CrossRef]
- Angioi, S.A.; Rau, D.; Attene, G.; Nanni, L.; Bellucci, E.; Logozzo, G.; Negri, V.; Spagnoletti Zeuli, P.L.; Papa, R. Beans in Europe: Origin and structure of the European landraces of Phaseolus vulgaris L. Theor. Appl. Genet. 2010, 121, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Piergiovanni, A.R.; Lioi, L. Italian common bean landraces: History, genetic diversity and seed quality. Diversity 2010, 2, 837–862. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#home (accessed on 15 June 2020).
- Blair, M.W.; González, L.F.; Kimani, P.M.; Butare, L. Genetic diversity, inter-gene pool introgression and nutritional quality of common beans (Phaseolus vulgaris L.) from Central Africa. Theor. Appl. Genet. 2010, 121, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Loveless, M.D.; Hamrick, J.L. Ecological determinants of genetic structure in plant populations. Annu. Rev. Ecol. Syst. 1984, 15, 65–95. [Google Scholar] [CrossRef]
- Linhart, Y.B.; Grant, M.C. Evolutionary significance of local genetic differentiation in plants. Annu. Rev. Ecol. Syst. 1996, 27, 237–277. [Google Scholar] [CrossRef]
- Schoen, D.J.; Brown, A.H. Intraspecific variation in population gene diversity and effective population size correlates with the mating system in plants. Proc. Natl. Acad. Sci. USA 1991, 88, 4494–4497. [Google Scholar] [CrossRef] [Green Version]
- Hurlbert, S.H. The nonconcept of species diversity: A critique and alternative parameters. Ecology 1971, 52, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Petit, R.J.; El Mousadik, A.; Pons, O. Identifying populations for conservation on the basis of genetic markers. Conserv. Biol. 1998, 12, 844–855. [Google Scholar] [CrossRef]
- de Souza, Y.G.; Greenspan, J.S. Biobanking past; present and future: Responsibilities and benefits. AIDS 2013, 28, 303–312. [Google Scholar] [CrossRef] [PubMed]
- van Treuren, R.; de Groot, E.C.; van Hintum, T.J.L. Preservation of seed viability during 25 years of storage under standard genebank conditions. Genet. Resour. Crop Evol. 2013, 60, 1407–1421. [Google Scholar] [CrossRef]
- FAO. Global Plan of Action for the Conservation and Sustainable Utilization of Plant Genetic Resources for Food and Agriculture and the Leipzig Declaration. In Proceedings of the International Technical Conference on Plant Genetic Resources, Leipzig, Germany, 17–23 June 1996. [Google Scholar]
- Beckman, H.B.; Franckel, R.M. The Effect of Physician Behavior on the Collection of Data. Ann. Intern. Med. 1984, 101, 692–696. [Google Scholar] [CrossRef]
- Brown, A.H.D. Core collections: A practical approach to genetic resources management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- van Hintum, T.J.L.; Brown, A.H.D.; Spillane, C. Core Collections of Plant Genetic Resources; Biodiversity International: Rome, Italy, 2000; p. 48. [Google Scholar]
- Díaz, L.M.; Blair, M.W. Race structure within the Mesoamerican gene pool of common bean (Phaseolus vulgaris L.) as determined by microsatellite markers. Theor. Appl. Genet. 2006, 114, 143–154. [Google Scholar] [CrossRef]
- Logozzo, G.; Donnoli, R.; Macaluso, L.; Papa, R.; Knüpffer, H.; Spagnoletti Zeuli, P.L. Analysis of the contribution of Mesoamerican and Andean gene pools to European common bean (Phaseolus vulgaris L.) germplasm and strategies to establish a core collection. Genet. Resour. Crop Evol. 2007, 54, 1763–1779. [Google Scholar] [CrossRef]
- Blair, M.W.; Díaz, L.M.; Buendía, H.F.; Duque, M.C. Genetic diversity, seed size associations and population structure of a core collection of common beans (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 119, 955–972. [Google Scholar] [CrossRef]
- Angioi, S.A.; Rau, D.; Rodriguez, M.; Logozzo, G.; Desiderio, F.; Papa, R.; Attene, G. Nuclear and chloroplast microsatellite diversity in Phaseolus vulgaris L. from Sardinia (Italy). Mol. Breed. 2009, 23, 413–429. [Google Scholar] [CrossRef]
- Perseguini, J.M.K.G.; Silva, G.M.B.; Rosa, J.R.B.F.; Gazaffi, R.; Marçal, J.F.; Carbonell, S.A.M.; Chiorat, A.F.; Zucchi, M.I.; Garcia, A.A.F.; Benchimol-Reis, V. Developing a common bean core collection suitable for association mapping studies. Genet. Mol. Biol. 2015, 38, 67–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitão, S.T.; Dinis, M.; Veloso, M.M.; Šatović, Z.; Vaz Patto, M.C. Establishing the bases for introducing the unexplored portuguese common bean germplasm into the breeding world. Front. Plant Sci. 2017, 8, 1296. [Google Scholar] [CrossRef] [Green Version]
- Bacchi, M.; Leone, M.; Mercati, F.; Preiti, G.; Sunseri, F.; Monti, M. Agronomic evaluation and genetic characterization of different accessions in lentil (Lens culinaris Medik.). Ital. J. Agron. 2010, 4, 303–314. [Google Scholar] [CrossRef]
- Gómez-Baggethun, E.; Corbera, E.; Reyes-García, V. Traditional ecological knowledge and global environmental change: Research findings and policy implications. Ecol. Soc. 2013, 18, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scialabba, A.; Bartolotta, I.; Digangi, I.; Geraci, M.; Raimondo, F.M.; Spadaro, V. The Botanical Garden “Bernardino da Ucria” in the Natural Park of the Nebrodi (Sicily) and its mission to conserve, exploit and spread local agrobiodiversity and officinal plants. In III International Plant Science Conference (IPSC)-111 Congresso Società Botanica Italiana; Società Botanica Italiana: Rome, Italy, 2016; p. 62. [Google Scholar]
- Scialabba, A.; Raimondo, F.M. La banca del germoplasma dell’Orto Botanico dell’Università di Palermo: Prime esperienze. Inform. Bot. Ital. 1994, 26, 176–183. [Google Scholar]
- Scialabba, A.; Raimondo, F.M. The “Sicilian Plant Germplasm Repository” of the University of Palermo: 25 years of activity in biological conservation. Bocconea 2019, 28, 391–392. [Google Scholar] [CrossRef]
- Harlan, J.R. Our vanishing genetic. Science 1975, 188, 617–621. [Google Scholar] [CrossRef]
- van Treuren, R.; van Hintum, T.J. Marker-assisted reduction of redundancy in germplasm collections: Genetic and economic aspects. Acta Hortic. 2003, 623, 139–149. [Google Scholar] [CrossRef] [Green Version]
- International Board for Plant Genetic Resources (IBPGR/IPGRI) Biodiversity International. In Phaseolus Vulgaris Descriptors; IBPGR: Rome, Italy, 1982.
- Biodiversity International, Rome (Italy/Centro Internacional de Agricultura Tropical (BI/CIAT). Key Access and Utilization Descriptors for Bean Genetic Resources. 2009. Available online: https://www.bioversityinternational.org/ (accessed on 18 June 2020).
- de Luca, D.; Cennamo, P.; del Guacchio, E.; di Novella, R.; Caputo, P. Conservation and genetic characterization of common bean landraces from Cilento region (southern Italy): High differentiation in spite of low genetic diversity. Genetica 2018, 146, 29–44. [Google Scholar] [CrossRef]
- Mercati, F.; Leone, M.; Lupini, A.; Sorgona, A.; Bacchi, M.; Abenavoli, M.R.; Sunseri, F. Genetic diversity and population structure of a common bean (Phaseolus vulgaris L.) collection from Calabria (Italy). Genet. Resour. Crop. Evol. 2013, 60, 839–852. [Google Scholar] [CrossRef]
- Scarano, D.; Rubio, F.; Ruiz, J.J.; Rao, R.; Giandomenico, C. Morphological and genetic diversity among and within common bean (Phaseolus vulgaris L.) landraces from the Campania region (Southern Italy). Sci. Hortic. 2014, 180, 72–78. [Google Scholar] [CrossRef]
- Buso, G.S.C.; Amaral, Z.P.S.; Brondani, R.P.V.; Ferreira, M.E. Microsatellite markers for the common bean Phaseolus vulgaris. Mol. Ecol. Notes 2006, 6, 252–254. [Google Scholar] [CrossRef]
- Burle, M.L.; Fonseca, J.R.; Kami, J.A.; Gepts, P. Microsatellite diversity and genetic structure among common bean (Phaseolus vulgaris L.) landraces in Brazil; a secondary center of diversity. Theor. Appl. Genet 2010, 121, 801–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, J.C.; Sharma, T.R.; Tyagi, R.K.; Chahota, R.K.; Gautam, N.K.; Mohar, S.; Sharma, P.N.; Ojha, S.N. Characterisation of 4274 accessions of common bean (Phaseolus vulgaris L.) germplasm conserved in the Indian gene bank for phenological, morphological and agricultural traits. Euphytica 2015, 205, 441–457. [Google Scholar] [CrossRef]
- Chiorato, A.F.; Carbonell, S.A.M.; Benchimol, L.L.; Chiavegato, M.B.; Dias, L.A.S.; Colombo, C.A. Genetic diversity in common bean accessions evaluated by means of morpho-agronomical and RAPD data. Sci. Agric. 2007, 64, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Lioi, L.; Nuzzi, A.; Campion, B.; Piergiovanni, A. Assessment of genetic variation in common bean (Phaseolus vulgaris L.) from Nebrodi mountains (Sicily, Italy). Genet. Resour. Crop Evol. 2012, 59, 455–464. [Google Scholar] [CrossRef]
- Raggi, L.; Tiranti, B.; Negri, V. Italian common bean landraces: Diversity and population structure. Genet. Resour. Crop Evol. 2013, 60, 1515–1530. [Google Scholar] [CrossRef]
- Paniconi, G.; Gianfilippi, F.; Mosconi, P.; Mazzucato, A. Distinctiveness of bean landraces in Italy: The case study of the ‘Badda’ bean. Diversity 2010, 2, 701–716. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, J.E. Genetic structure of landraces. In Plant Breeding: Past, Present and Future; Springer International Publishing: Cham, Switzerland, 2016; pp. 273–290. [Google Scholar] [CrossRef]
- Carucci, F.; Garramone, R.; Aversano, R.; Carputo, D. SSR markers distinguish traditional Italian bean (Phaseolus vulgaris L.) landraces from Lamon. Czech J. Genet. Plant Breed. 2017, 53, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Pipan, B.; Meglič, V. Diversification and genetic structure of the western-to-eastern progression of European Phaseolus vulgaris L. germplasm. BMC Plant Biol. 2019, 19, 442. [Google Scholar] [CrossRef]
- Sarıkamış, G.; Yaşar, F.; Bakır, M.; Kazan, K.; Ergül, A. Genetic characterization of green bean (Phaseolus vulgaris) genotypes from eastern Turkey. Genet. Mol. Res. 2009, 8, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Carović-Stanko, K.; Liber, Z.; Vidak, M.; Barešić, A.; Grdiša, M.; Lazarević, B.; Šatović, Z. Genetic Diversity of Croatian Common Bean Landraces. Front. Plant Sci. 2017, 8, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maras, M.; Pipan, B.; Sustar-Vozlic, J.; Todorović, V.; Đurić, G.; Vasić, M.; Kratovalieva, S.; Ibusoska, A.; Agić, R.; Matotan, Z.; et al. Examination of genetic diversity of common bean from the Western Balkans. J. Am. Soc. Hort. Sci. 2015, 140, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, S.T. Counting alleles with rarefaction: Private alleles and hierarchical sampling designs. Conserv. Genet. 2004, 5, 539–543. [Google Scholar] [CrossRef]
- Gioia, T.; Logozzo, G.; Marzario, S.; Spagnoletti Zeuli, P.L.; Gepts, P. Evolution of SSR diversity from wild types to U.S. advanced cultivars in the Andean and Mesoamerican domestications of common bean (Phaseolus vulgaris). PLoS ONE 2019, 14, e0211342. [Google Scholar] [CrossRef] [Green Version]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus ssp.) as a Model for Understanding Crop Evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef] [Green Version]
- Caproni, L.; Raggi, L.; Ceccarelli, S.; Negri, V.; Carboni, A. In-depth characterisation of common bean diversity discloses its breeding potential for sustainable agriculture. Sustainability 2019, 11, 5443. [Google Scholar] [CrossRef] [Green Version]
- Gepts, P. A Middle American and an Andean Common Bean Gene Pool. In Genetic Resources of Phaseolus Beans. Current Plant Science and Biotechnology in Agriculture; Gepts, P., Ed.; Springer: Dordrecht, The Netherlands, 1988; p. 6. [Google Scholar] [CrossRef]
- Kwak, M.; Toro, O.; Debouck, D.G.; Gepts, P. Multiple origins of the determinate growth habit in domesticated common bean (Phaseolus vulgaris). Ann. Bot. 2012, 110, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Casquero, P.A.; Lema, M.; Santalla, M.; de Ron, A.M. Performance of Common Bean (Phaseolus vulgaris L.) Landraces from Spain in the Atlantic and Mediterranean Environments. Genet. Resour. Crop. Evol. 2006, 53, 1021–1032. [Google Scholar] [CrossRef] [Green Version]
- Assefa, T.; Wu, J.; Beebe, S.E.; Rao, J.M.; Marcomin, D.; Claude, R.J. Improving adaptation to drought stress in small red common bean: Phenotypic differences and predicted genotypic effects on grain yield, yield components and harvest index. Euphytica 2015, 203, 477–489. [Google Scholar] [CrossRef]
- Darkwa, K.; Ambachewa, D.; Mohammed, H.; Asfawa, A.; Blair, M.W. Evaluation of common bean (Phaseolus vulgaris L.) genotypes for drought stress adaptation in Ethiopia. Crop. J. 2016, 4, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Scialabba, A.; Raimondo, F.M. Hortus Botanicus Panormitanus seed bank. Studi Trent. Sci. Nat. 2012, 30, 93–97. [Google Scholar]
- Yu, K.; Park, S.J.; Poysa, V.; Gepts, P. Integration of simple sequence repeat (SSR) markers into a molecular linkage map of common bean (Phaseolus vulgaris L.). J. Hered. 2000, 91, 429–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaitán-Solís, E.; Duque, M.C.; Edwards, K.J.; Tohme, J. Microsatellite repeats in common bean (Phaseolus vulgaris L.): Isolation, characterization, and cross-species amplification in Phaseolus ssp. Crop. Sci. 2002, 42, 2128–2136. [Google Scholar] [CrossRef]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Marshal, D.R.; Brown, A.H.D. Optimum sampling strategies in genetic conservation. In Crop Genetic Resources for Today and Tomorrow; Frankel, O.H., Hawkes, J.G., Eds.; Cambridge University Press: London, UK, 1975; pp. 53–58. [Google Scholar]
- Peakall, R.; Smouse, P.E. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [Green Version]
- Bruvo, R.; Michiels, N.K.; D’souza, T.G.; Schulenburg, H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef]
- Jombart, T.; Ahmed, I. Adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [PubMed]
- Mercati, F.; Longo, C.; Poma, D.; Araniti, F.; Lupini, A.; Mammano, M.M.; Fiore, M.C.; Abenavoli, M.R.; Sunseri, F. Genetic variation of an Italian long shelf-life tomato (Solanum lycopersicon L.) collection by using SSR and morphological fruit traits. Genet. Resour. Crop. Evol. 2015, 62, 721–732. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar] [PubMed]
- Goosle, S.C.; Urban, D.L. The ecodist Package for Dissimilarity-based Analysis of Ecological Data. J. Stat. Softw. 2007, 22, 1–19. [Google Scholar] [CrossRef]






| Locus | Na | Range Size (bp) | Ho | He | PIC | PI | Hom | PD |
|---|---|---|---|---|---|---|---|---|
| PVag001 | 6 | 137–155 | 0.333 | 0.524 | 0.463 | 0.288 | 66.7 | 0.7120 |
| GATS91 | 11 | 214–256 | 0.035 | 0.733 | 0.709 | 0.0916 | 96.5 | 0.9084 |
| BM210 | 9 | 167–195 | 0.0350 | 0.746 | 0.713 | 0.0972 | 98.2 | 0.9028 |
| BM160 | 12 | 180–256 | 0.019 | 0.867 | 0.853 | 0.0318 | 98.2 | 0.9682 |
| BM159 | 5 | 187–199 | 0.123 | 0.656 | 0.593 | 0.181 | 91.2 | 0.8189 |
| PVBR25 | 13 | 149–177 | 0.182 | 0.802 | 0.785 | 0.0564 | 82.5 | 0.9436 |
| BM172 | 9 | 70–112 | 0.089 | 0.636 | 0.590 | 0.1687 | 94.7 | 0.8313 |
| PVBR163 | 10 | 221–259 | 0.439 | 0.646 | 0.607 | 0.1606 | 56.1 | 0.8394 |
| Overall | 9.375 a | - | 0.157 a | 0.701 a | 0.664 a | 2 × 10−8 b | 85.5 a | 0.8656 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M.C.; Raimondo, F.M.; Mercati, F.; Digangi, I.; Sunseri, F.; Scialabba, A. Preserving Biodiversity in Marginal Rural Areas: Assessment of Morphological and Genetic Variability of a Sicilian Common Bean Germplasm Collection. Plants 2020, 9, 989. https://doi.org/10.3390/plants9080989
Fiore MC, Raimondo FM, Mercati F, Digangi I, Sunseri F, Scialabba A. Preserving Biodiversity in Marginal Rural Areas: Assessment of Morphological and Genetic Variability of a Sicilian Common Bean Germplasm Collection. Plants. 2020; 9(8):989. https://doi.org/10.3390/plants9080989
Chicago/Turabian StyleFiore, Maria Carola, Francesco Maria Raimondo, Francesco Mercati, Ignazio Digangi, Francesco Sunseri, and Anna Scialabba. 2020. "Preserving Biodiversity in Marginal Rural Areas: Assessment of Morphological and Genetic Variability of a Sicilian Common Bean Germplasm Collection" Plants 9, no. 8: 989. https://doi.org/10.3390/plants9080989