Extracellular Vesicles and MicroRNA: Putative Role in Diagnosis and Treatment of Diabetic Retinopathy
Abstract
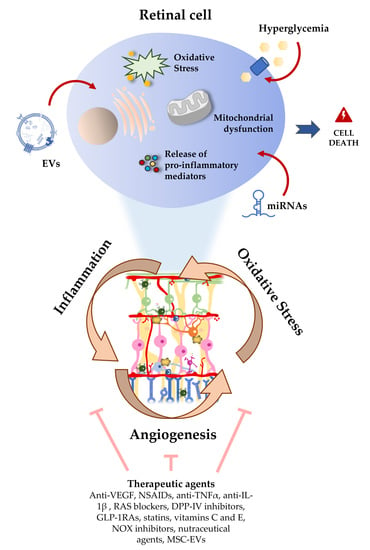
:1. Introduction
2. Pathophysiologic Changes and Clinical Definition of DR
3. The Role of Oxidative Stress and Inflammation in DR
4. Crosstalk between Endothelial Cells and Other Retinal Cells through Extracellular Vesicles (EVs)
5. Contribution of Plasma EVs to Microvascular Damage in DR
6. miRNA in DR: Role in Oxidative Stress and Inflammatory Signaling Pathways
7. Therapeutic and Nutraceutical Agents with Anti-Inflammatory and Antioxidant Properties against DR
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Federation, I.D. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- Wang, P.; Chen, F.; Wang, W.; Zhang, X.D. Hydrogen Sulfide Attenuates High Glucose-Induced Human Retinal Pigment Epithelial Cell Inflammation by Inhibiting ROS Formation and NLRP3 Inflammasome Activation. Mediat. Inflamm. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Youngblood, H.; Robinson, R.; Sharma, A.; Sharma, S. Proteomic Biomarkers of Retinal Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2019, 20, 4755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Sarabusky, M.; Chakrabarti, S. Diabetic Retinopathy, lncRNAs, and Inflammation: A Dynamic, Interconnected Network. J. Clin. Med. 2019, 8, 1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simo, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, M.L.; Perez, S.; Mena-Molla, S.; Desco, M.C.; Ortega, A.L. Oxidative Stress and Microvascular Alterations in Diabetic Retinopathy: Future Therapies. Oxid. Med. Cell Longev. 2019, 2019, 4940825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, D.S.; Aiello, L.P.; Ferris, F.L., 3rd; Klein, R. Diabetic retinopathy. Diabetes Care 2004, 27, 2540–2553. [Google Scholar] [CrossRef] [Green Version]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013, 2013, 343560. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. (Lausanne) 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Yu, Z.W.; Wang, Y.; Fu, Y.H.; Gao, X.Y. MicroRNAs: Potential Targets in Diabetic Retinopathy. Horm. Metab. Res. 2020, 52, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.Y.; Cheung, C.M.; Larsen, M.; Sharma, S.; Simo, R. Diabetic retinopathy. Nat. Rev. Dis. Primers 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.L.; Lin, C.H.; Li, C.H.; Tsai, C.H.; Ho, J.D.; Chiou, G.C.; Kang, J.J.; Cheng, Y.W. Anti-inflammatory properties of shikonin contribute to improved early-stage diabetic retinopathy. Sci. Rep. 2017, 7, 44985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef]
- Gardner, T.W.; Abcouwer, S.F.; Barber, A.J.; Jackson, G.R. An integrated approach to diabetic retinopathy research. Arch. Ophthalmol. 2011, 129, 230–235. [Google Scholar] [CrossRef] [Green Version]
- de Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Gallego, B.I.; Trivino, A.; Ramirez, J.M. Retinal Macroglial Responses in Health and Disease. Biomed. Res. Int. 2016, 2016, 2954721. [Google Scholar] [CrossRef] [Green Version]
- Bresnick, G.H. Diabetic retinopathy viewed as a neurosensory disorder. Arch. Ophthalmol. 1986, 104, 989–990. [Google Scholar] [CrossRef]
- Ghirlanda, G.; Di Leo, M.A.; Caputo, S.; Cercone, S.; Greco, A.V. From functional to microvascular abnormalities in early diabetic retinopathy. Diabetes Metab. Rev. 1997, 13, 15–35. [Google Scholar] [CrossRef]
- Greenstein, V.C.; Shapiro, A.; Zaidi, Q.; Hood, D.C. Psychophysical evidence for post-receptoral sensitivity loss in diabetics. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2781–2790. [Google Scholar]
- Antonetti, D.A.; Barber, A.J.; Bronson, S.K.; Freeman, W.M.; Gardner, T.W.; Jefferson, L.S.; Kester, M.; Kimball, S.R.; Krady, J.K.; LaNoue, K.F.; et al. Diabetic retinopathy: Seeing beyond glucose-induced microvascular disease. Diabetes 2006, 55, 2401–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in Type 2 Diabetes: Evidence From Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333–6338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic Microvascular Disease: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabrawey, M.; Zhang, W.; McDonald, D. Diabetic retinopathy: Mechanism, diagnosis, prevention, and treatment. Biomed. Res. Int. 2015, 2015, 854593. [Google Scholar] [CrossRef]
- Rubsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [Green Version]
- Othman, R.; Vaucher, E.; Couture, R. Bradykinin Type 1 Receptor—Inducible Nitric Oxide Synthase: A New Axis Implicated in Diabetic Retinopathy. Front. Pharmacol. 2019, 10, 300. [Google Scholar] [CrossRef]
- Sorrentino, F.S.; Matteini, S.; Bonifazzi, C.; Sebastiani, A.; Parmeggiani, F. Diabetic retinopathy and endothelin system: Microangiopathy versus endothelial dysfunction. Eye (Lond.) 2018, 32, 1157–1163. [Google Scholar] [CrossRef] [Green Version]
- Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology 1991, 98, 766–785. [Google Scholar] [CrossRef]
- Santiago, A.R.; Boia, R.; Aires, I.D.; Ambrosio, A.F.; Fernandes, R. Sweet Stress: Coping With Vascular Dysfunction in Diabetic Retinopathy. Front. Physiol. 2018, 9, 820. [Google Scholar] [CrossRef]
- Abu El-Asrar, A.M.; Ahmad, A.; Siddiquei, M.M.; De Zutter, A.; Allegaert, E.; Gikandi, P.W.; De Hertogh, G.; Van Damme, J.; Opdenakker, G.; Struyf, S. The Proinflammatory and Proangiogenic Macrophage Migration Inhibitory Factor Is a Potential Regulator in Proliferative Diabetic Retinopathy. Front. Immunol. 2019, 10, 2752. [Google Scholar] [CrossRef] [Green Version]
- Aouiss, A.; Anka Idrissi, D.; Kabine, M.; Zaid, Y. Update of inflammatory proliferative retinopathy: Ischemia, hypoxia and angiogenesis. Curr. Res. Transl. Med. 2019, 67, 62–71. [Google Scholar] [CrossRef]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [Green Version]
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Yumnamcha, T.; Devi, T.S.; Singh, L.P. Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1065. [Google Scholar] [CrossRef] [PubMed]
- Kooragayala, K.; Gotoh, N.; Cogliati, T.; Nellissery, J.; Kaden, T.R.; French, S.; Balaban, R.; Li, W.; Covian, R.; Swaroop, A. Quantification of Oxygen Consumption in Retina Ex Vivo Demonstrates Limited Reserve Capacity of Photoreceptor Mitochondria. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8428–8436. [Google Scholar] [CrossRef] [Green Version]
- Hombrebueno, J.R.; Cairns, L.; Dutton, L.R.; Lyons, T.J.; Brazil, D.P.; Moynagh, P.; Curtis, T.M.; Xu, H. Uncoupled turnover disrupts mitochondrial quality control in diabetic retinopathy. JCI Insight 2019, 4, e129760. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [Green Version]
- Berezin, A. Neutrophil extracellular traps: The core player in vascular complications of diabetes mellitus. Diabetes Metab. Syndr. 2019, 13, 3017–3023. [Google Scholar] [CrossRef]
- Geraldes, P.; King, G.L. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ. Res. 2010, 106, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, M.; Barber, A.J.; Antonetti, D.A.; LaNoue, K.F.; Robinson, K.A.; Buse, M.G.; Gardner, T.W. Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J. Biol. Chem. 2001, 276, 43748–43755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowluru, R.A.; Mishra, M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim. Biophys. Acta 2015, 1852, 2474–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Matsumura, T.; Edelstein, D.; Rossetti, L.; Zsengeller, Z.; Szabo, C.; Brownlee, M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J. Clin. Investig. 2003, 112, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Adhya, P.; Sharma, S.S. Redox TRPs in diabetes and diabetic complications: Mechanisms and pharmacological modulation. Pharmacol. Res. 2019, 146, 104271. [Google Scholar] [CrossRef]
- Abcouwer, S.F. Muller Cell-Microglia Cross Talk Drives Neuroinflammation in Diabetic Retinopathy. Diabetes 2017, 66, 261–263. [Google Scholar] [CrossRef] [Green Version]
- Rangasamy, S.; McGuire, P.G.; Franco Nitta, C.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine mediated monocyte trafficking into the retina: Role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef]
- Semeraro, F.; Cancarini, A.; dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Howell, S.J.; Hatala, D.A.; Huang, K.; Kern, T.S. Salicylate-based anti-inflammatory drugs inhibit the early lesion of diabetic retinopathy. Diabetes 2007, 56, 337–345. [Google Scholar] [CrossRef] [Green Version]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M. Matrix metalloproteinases in diabetic retinopathy: Potential role of MMP-9. Expert Opin. Investig. Drugs 2012, 21, 797–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, G.; Kowluru, R.A. Novel role of mitochondrial matrix metalloproteinase-2 in the development of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3832–3841. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.D.; Distefano, G.; Gagliano, C.; Rusciano, D.; Malaguarnera, L. Autophagy in Diabetic Retinopathy. Curr. Neuropharmacol. 2016, 14, 810–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picconi, F.; Parravano, M.; Ylli, D.; Pasqualetti, P.; Coluzzi, S.; Giordani, I.; Malandrucco, I.; Lauro, D.; Scarinci, F.; Giorno, P.; et al. Retinal neurodegeneration in patients with type 1 diabetes mellitus: The role of glycemic variability. Acta Diabetol. 2017, 54, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef] [Green Version]
- Hauser, P.; Wang, S.; Didenko, V.V. Apoptotic Bodies: Selective Detection in Extracellular Vesicles. Methods Mol. Biol. 2017, 1554, 193–200. [Google Scholar]
- van der Merwe, Y.; Steketee, M.B. Extracellular Vesicles: Biomarkers, Therapeutics, and Vehicles in the Visual System. Curr. Ophthalmol. Rep. 2017, 5, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Mighty, J.; Zhou, J.; Benito-Martin, A.; Sauma, S.; Hanna, S.; Onwumere, O.; Shi, C.; Muntzel, M.; Sauane, M.; Young, M.; et al. Analysis of Adult Neural Retina Extracellular Vesicle Release, RNA Transport and Proteomic Cargo. Investig. Ophthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef] [Green Version]
- Hajrasouliha, A.R.; Jiang, G.; Lu, Q.; Lu, H.; Kaplan, H.J.; Zhang, H.G.; Shao, H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem. 2013, 288, 28058–28067. [Google Scholar] [CrossRef] [Green Version]
- Klingeborn, M.; Dismuke, W.M.; Skiba, N.P.; Kelly, U.; Stamer, W.D.; Bowes Rickman, C. Directional Exosome Proteomes Reflect Polarity-Specific Functions in Retinal Pigmented Epithelium Monolayers. Sci. Rep. 2018, 8, 17327. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.Y.; Bang, J.Y.; Choi, A.J.; Yoon, J.; Lee, W.C.; Choi, S.; Yoon, S.; Kim, H.C.; Baek, J.H.; Park, H.S.; et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J. Proteome Res. 2014, 13, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Lerner, N.; Avissar, S.; Beit-Yannai, E. Extracellular vesicles mediate signaling between the aqueous humor producing and draining cells in the ocular system. PLoS ONE 2017, 12, e0171153. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Contreras, M.; Brooks, R.W.; Boccuzzi, L.; Robbins, P.D.; Ricordi, C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2940–2956. [Google Scholar]
- Zhou, F.; Huang, L.; Qu, S.L.; Chao, R.; Yang, C.; Jiang, Z.S.; Zhang, C. The emerging roles of extracellular vesicles in diabetes and diabetic complications. Clin. Chim. Acta 2019, 497, 130–136. [Google Scholar] [CrossRef]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Extracellular vesicles have variable dose-dependent effects on cultured draining cells in the eye. J. Cell Mol. Med. 2018, 22, 1992–2000. [Google Scholar] [CrossRef]
- Chahed, S.; Leroyer, A.S.; Benzerroug, M.; Gaucher, D.; Georgescu, A.; Picaud, S.; Silvestre, J.S.; Gaudric, A.; Tedgui, A.; Massin, P.; et al. Increased vitreous shedding of microparticles in proliferative diabetic retinopathy stimulates endothelial proliferation. Diabetes 2010, 59, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ge, H.M.; Liu, B.H.; Dong, R.; Shan, K.; Chen, X.; Yao, M.D.; Li, X.M.; Yao, J.; Zhou, R.M.; et al. Targeting pericyte-endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 7455–7464. [Google Scholar] [CrossRef] [Green Version]
- Beltramo, E.; Lopatina, T.; Berrone, E.; Mazzeo, A.; Iavello, A.; Camussi, G.; Porta, M. Extracellular vesicles derived from mesenchymal stem cells induce features of diabetic retinopathy in vitro. Acta Diabetol. 2014, 51, 1055–1064. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Liu, M.L. Pathogenic roles of microvesicles in diabetic retinopathy. Acta Pharmacol. Sin. 2018, 39, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vitkova, V.; Zivny, J.; Janota, J. Endothelial cell-derived microvesicles: Potential mediators and biomarkers of pathologic processes. Biomark. Med. 2018, 12, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Bastiaans, J.; van Meurs, J.C.; Mulder, V.C.; Nagtzaam, N.M.; Smits-te Nijenhuis, M.; Dufour-van den Goorbergh, D.C.; van Hagen, P.M.; Hooijkaas, H.; Dik, W.A. The role of thrombin in proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4659–4666. [Google Scholar] [CrossRef]
- Huang, H.; Gandhi, J.K.; Zhong, X.; Wei, Y.; Gong, J.; Duh, E.J.; Vinores, S.A. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1336–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial Microparticle Formation by Angiotensin II Is Mediated via Ang II Receptor Type I/NADPH Oxidase/Rho Kinase Pathways Targeted to Lipid Rafts. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1898–1907. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Wu, Y.; Hu, Z.; Sun, L.; Dou, G.; Zhang, Z.; Wang, H.; Guo, C.; Wang, Y. Exosomes from Microglia Attenuate Photoreceptor Injury and Neovascularization in an Animal Model of Retinopathy of Prematurity. Mol. Ther. Nucleic Acids 2019, 16, 778–790. [Google Scholar] [CrossRef] [Green Version]
- Tokarz, A.; Szuscik, I.; Kusnierz-Cabala, B.; Kapusta, M.; Konkolewska, M.; Zurakowski, A.; Georgescu, A.; Stepien, E. Extracellular vesicles participate in the transport of cytokines and angiogenic factors in diabetic patients with ocular complications. Folia Med. Cracov. 2015, 55, 35–48. [Google Scholar]
- Tsimerman, G.; Roguin, A.; Bachar, A.; Melamed, E.; Brenner, B.; Aharon, A. Involvement of microparticles in diabetic vascular complications. Thromb. Haemost. 2011, 106, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chen, M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur. J. Pharmacol. 2016, 787, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrzanowska, M.; Modrzejewska, A.; Modrzejewska, M. New insight into the role of the complement in the most common types of retinopathy-current literature review. Int. J. Ophthalmol. 2018, 11, 1856–1864. [Google Scholar]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma Exosomes Contribute to Microvascular Damage in Diabetic Retinopathy by Activating the Classical Complement Pathway. Diabetes 2018, 67, 1639–1649. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Busik, J.V. Extracellular Vesicle-Induced Classical Complement Activation Leads to Retinal Endothelial Cell Damage via MAC Deposition. Int. J. Mol. Sci. 2020, 21, 1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerl, V.B.; Bohl, J.; Pitz, S.; Stoffelns, B.; Pfeiffer, N.; Bhakdi, S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1104–1108. [Google Scholar]
- Ogata, N.; Nomura, S.; Shouzu, A.; Imaizumi, M.; Arichi, M.; Matsumura, M. Elevation of monocyte-derived microparticles in patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 2006, 73, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ogata, N.; Imaizumi, M.; Nomura, S.; Shozu, A.; Arichi, M.; Matsuoka, M.; Matsumura, M. Increased levels of platelet-derived microparticles in patients with diabetic retinopathy. Diabetes Res. Clin. Pract. 2005, 68, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, X.; Wang, T.; Kong, Y. Exosomes derived from platelet-rich plasma mediate hyperglycemia-induced retinal endothelial injury via targeting the TLR4 signaling pathway. Exp. Eye Res. 2019, 189, 107813. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, A.; Konkolewska, M.; Kusnierz-Cabala, B.; Maziarz, B.; Hanarz, P.; Zurakowski, A.; Szuscik, I.; Stepien, E.L. Retinopathy severity correlates with RANTES concentrations and CCR 5-positive microvesicles in diabetes. Folia Med. Cracov. 2019, 59, 95–112. [Google Scholar]
- Jansen, F.; Yang, X.; Franklin, B.S.; Hoelscher, M.; Schmitz, T.; Bedorf, J.; Nickenig, G.; Werner, N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc. Res. 2013, 98, 94–106. [Google Scholar] [CrossRef] [Green Version]
- Razmara, M.; Hjemdahl, P.; Ostenson, C.G.; Li, N. Platelet hyperprocoagulant activity in Type 2 diabetes mellitus: Attenuation by glycoprotein IIb/IIIa inhibition. J. Thromb. Haemost. 2008, 6, 2186–2192. [Google Scholar] [CrossRef]
- Su, Y.; Chen, J.; Dong, Z.; Zhang, Y.; Ma, R.; Kou, J.; Wang, F.; Shi, J. Procoagulant Activity of Blood and Endothelial Cells via Phosphatidylserine Exposure and Microparticle Delivery in Patients with Diabetic Retinopathy. Cell Physiol. Biochem. 2018, 45, 2411–2420. [Google Scholar] [CrossRef]
- Hausser, J.; Zavolan, M. Identification and consequences of miRNA-target interactions--beyond repression of gene expression. Nat. Rev. Genet. 2014, 15, 599–612. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef]
- Bushati, N.; Cohen, S.M. microRNA functions. Annu. Rev. Cell Dev. Biol. 2007, 23, 175–205. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, K.U.; Oner, M.G.; Meuwissen, R.L.; Genc, S. The role of microRNAs in human diseases. Methods Mol. Biol. 2014, 1107, 33–50. [Google Scholar] [PubMed]
- Konovalova, J.; Gerasymchuk, D.; Parkkinen, I.; Chmielarz, P.; Domanskyi, A. Interplay between MicroRNAs and Oxidative Stress in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 6055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafabakhsh, R.; Aghadavod, E.; Mobini, M.; Heidari-Soureshjani, R.; Asemi, Z. Association between microRNAs expression and signaling pathways of inflammatory markers in diabetic retinopathy. J. Cell Physiol. 2019, 234, 7781–7787. [Google Scholar] [CrossRef]
- Garcia de la Torre, N.; Fernandez-Durango, R.; Gomez, R.; Fuentes, M.; Roldan-Pallares, M.; Donate, J.; Barabash, A.; Alonso, B.; Runkle, I.; Duran, A.; et al. Expression of Angiogenic MicroRNAs in Endothelial Progenitor Cells From Type 1 Diabetic Patients With and Without Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4090–4098. [Google Scholar] [CrossRef] [Green Version]
- Qing, S.; Yuan, S.; Yun, C.; Hui, H.; Mao, P.; Wen, F.; Ding, Y.; Liu, Q. Serum miRNA biomarkers serve as a fingerprint for proliferative diabetic retinopathy. Cell Physiol. Biochem. 2014, 34, 1733–1740. [Google Scholar] [CrossRef]
- Zampetaki, A.; Willeit, P.; Burr, S.; Yin, X.; Langley, S.R.; Kiechl, S.; Klein, R.; Rossing, P.; Chaturvedi, N.; Mayr, M. Angiogenic microRNAs Linked to Incidence and Progression of Diabetic Retinopathy in Type 1 Diabetes. Diabetes 2016, 65, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Yuan, M.; Liu, Y.; Zhao, X.; Lian, P.; Chen, Y.; Liu, B.; Lu, L. Landscape of microRNA in the aqueous humour of proliferative diabetic retinopathy as assessed by next-generation sequencing. Clin. Exp. Ophthalmol. 2019, 47, 925–936. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. MicroRNAs as biomarkers of diabetic retinopathy and disease progression. Neural Regen. Res. 2019, 14, 1858–1869. [Google Scholar]
- Jiang, Q.; Lyu, X.M.; Yuan, Y.; Wang, L. Plasma miR-21 expression: An indicator for the severity of Type 2 diabetes with diabetic retinopathy. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Peng, C.; Huang, D.S.; Liu, L.; Guan, P. microRNA Expression Profiling Based on Microarray Approach in Human Diabetic Retinopathy: A Systematic Review and Meta-Analysis. DNA Cell Biol. 2020, 39, 441–450. [Google Scholar] [CrossRef]
- Iftikhar, H.; Carney, G.E. Evidence and potential in vivo functions for biofluid miRNAs: From expression profiling to functional testing: Potential roles of extracellular miRNAs as indicators of physiological change and as agents of intercellular information exchange. Bioessays 2016, 38, 367–378. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [Green Version]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef]
- Ferreira, R.; Santos, T.; Amar, A.; Gong, A.; Chen, T.C.; Tahara, S.M.; Giannotta, S.L.; Hofman, F.M. Argonaute-2 promotes miR-18a entry in human brain endothelial cells. J. Am. Heart Assoc. 2014, 3, e000968. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zhang, S.; Weber, J.; Baxter, D.; Galas, D.J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010, 38, 7248–7259. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [Green Version]
- Sedgeman, L.R.; Beysen, C.; Ramirez Solano, M.A.; Michell, D.L.; Sheng, Q.; Zhao, S.; Turner, S.; Linton, M.F.; Vickers, K.C. Beta cell secretion of miR-375 to HDL is inversely associated with insulin secretion. Sci. Rep. 2019, 9, 3803. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Dong, Y.; He, C.; Pan, X.; Liu, D.; Yang, J.; Sun, L.; Chen, P.; Wang, Q. RNA-Seq Revealed Novel Non-proliferative Retinopathy Specific Circulating MiRNAs in T2DM Patients. Front. Genet. 2019, 10, 531. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Luo, J.; Zhang, H. Role of Sirtuin 1 in the pathogenesis of ocular disease (Review). Int. J. Mol. Med. 2018, 42, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008, 36, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Shaker, O.G.; Abdelaleem, O.O.; Mahmoud, R.H.; Abdelghaffar, N.K.; Ahmed, T.I.; Said, O.M.; Zaki, O.M. Diagnostic and prognostic role of serum miR-20b, miR-17-3p, HOTAIR, and MALAT1 in diabetic retinopathy. IUBMB Life 2019, 71, 310–320. [Google Scholar] [CrossRef]
- Liu, L.Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.F.; Lai, L.; Jiang, B.H. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS ONE 2011, 6, e19139. [Google Scholar]
- Zeng, J.; Xiong, Y.; Li, G.; Liu, M.; He, T.; Tang, Y.; Chen, Y.; Cai, L.; Jiang, R.; Tao, J. MiR-21 is overexpressed in response to high glucose and protects endothelial cells from apoptosis. Exp. Clin. Endocrinol. Diabetes 2013, 121, 425–430. [Google Scholar] [CrossRef]
- Solly, E.; Hourigan, S.T.; Mulangala, J.; Psaltis, P.J.; Di Bartolo, B.; NG, M.; Nicholas, S.; Bursill, C.; Tan, J.T.M. Inhibition of miR-181c Rescues Diabetes-Impaired Angiogenesis. Diabetes 2019, 68 (Suppl. 1). [Google Scholar] [CrossRef]
- McArthur, K.; Feng, B.; Wu, Y.; Chen, S.; Chakrabarti, S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes 2011, 60, 1314–1323. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.R.; Chen, Q.; Takahashi, Y.; Zhou, K.K.; Park, K.; Ma, J.X. MicroRNA-200b downregulates oxidation resistance 1 (Oxr1) expression in the retina of type 1 diabetes model. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1689–1697. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, B.; Lumayag, S.; Cowan, C.; Xu, S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4402–4409. [Google Scholar] [CrossRef]
- Chen, S.; Feng, B.; George, B.; Chakrabarti, R.; Chen, M.; Chakrabarti, S. Transcriptional coactivator p300 regulates glucose-induced gene expression in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2010, 298, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, J.; Steel, D.H.W.; Schlingemann, R.O.; Koss, M.J.; Hammes, H.P.; Krenning, G.; Klassen, I. microRNA Expression Profile in the Vitreous of Proliferative Diabetic Retinopathy Patients and Differences from Patients Treated with Anti-VEGF Therapy. Transl. Vis. Sci. Technol. 2020, 9, 16. [Google Scholar] [CrossRef]
- Pezzolesi, M.G.; Satake, E.; McDonnell, K.P.; Major, M.; Smiles, A.M.; Krolewski, A.S. Circulating TGF-beta1-Regulated miRNAs and the Risk of Rapid Progression to ESRD in Type 1 Diabetes. Diabetes 2015, 64, 3285–3293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.; Danielson, K.M.; Benton, M.C.; Ziegler, O.; Shah, R.; Stubbs, R.S.; Das, S.; Macartney-Coxson, D. miRNA Signatures of Insulin Resistance in Obesity. Obesity (Silver Spring) 2017, 25, 1734–1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meerson, A.; Najjar, A.; Saad, E.; Sbeit, W.; Barhoum, M.; Assy, N. Sex Differences in Plasma MicroRNA Biomarkers of Early and Complicated Diabetes Mellitus in Israeli Arab and Jewish Patients. Noncoding RNA 2019, 5, 32. [Google Scholar] [CrossRef] [Green Version]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lucena, R.; Rangel-Zuniga, O.A.; Alcala-Diaz, J.F.; Lopez-Moreno, J.; Roncero-Ramos, I.; Molina-Abril, H.; Yubero-Serrano, E.M.; Caballero-Villarraso, J.; Delgado-Lista, J.; Castano, J.P.; et al. Circulating miRNAs as Predictive Biomarkers of Type 2 Diabetes Mellitus Development in Coronary Heart Disease Patients from the CORDIOPREV Study. Mol. Ther. Nucleic Acids 2018, 12, 146–157. [Google Scholar] [CrossRef]
- Seyhan, A.A.; Nunez Lopez, Y.O.; Xie, H.; Yi, F.; Mathews, C.; Pasarica, M.; Pratley, R.E. Pancreas-enriched miRNAs are altered in the circulation of subjects with diabetes: A pilot cross-sectional study. Sci. Rep. 2016, 6, 31479. [Google Scholar] [CrossRef] [Green Version]
- de Candia, P.; Spinetti, G.; Specchia, C.; Sangalli, E.; La Sala, L.; Uccellatore, A.; Lupini, S.; Genovese, S.; Matarese, G.; Ceriello, A. A unique plasma microRNA profile defines type 2 diabetes progression. PLoS ONE 2017, 12, e0188980. [Google Scholar] [CrossRef] [Green Version]
- Hirota, K.; Keino, H.; Inoue, M.; Ishida, H.; Hirakata, A. Comparisons of microRNA expression profiles in vitreous humor between eyes with macular hole and eyes with proliferative diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 335–342. [Google Scholar] [CrossRef]
- Blum, A.; Yehuda, H.; Geron, N.; Meerson, A. Elevated Levels of miR-122 in Serum May Contribute to Improved Endothelial Function and Lower Oncologic Risk Following Bariatric Surgery. Isr. Med. Assoc. J. 2017, 19, 620–624. [Google Scholar]
- Pastukh, N.; Meerson, A.; Kalish, D.; Jabaly, H.; Blum, A. Serum miR-122 levels correlate with diabetic retinopathy. Clin. Exp. Med. 2019, 19, 255–260. [Google Scholar] [CrossRef]
- Wang, X.; Sundquist, J.; Zoller, B.; Memon, A.A.; Palmer, K.; Sundquist, K.; Bennet, L. Determination of 14 circulating microRNAs in Swedes and Iraqis with and without diabetes mellitus type 2. PLoS ONE 2014, 9, e86792. [Google Scholar] [CrossRef]
- Kong, L.; Zhu, J.; Han, W.; Jiang, X.; Xu, M.; Zhao, Y.; Dong, Q.; Pang, Z.; Guan, Q.; Gao, L.; et al. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: A clinical study. Acta Diabetol. 2011, 48, 61–69. [Google Scholar] [CrossRef]
- Flowers, E.; Kanaya, A.M.; Fukuoka, Y.; Allen, I.E.; Cooper, B.; Aouizerat, B.E. Preliminary evidence supports circulating microRNAs as prognostic biomarkers for type 2 diabetes. Obes. Sci. Pract. 2017, 3, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Carreras-Badosa, G.; Bonmati, A.; Ortega, F.J.; Mercader, J.M.; Guindo-Martinez, M.; Torrents, D.; Prats-Puig, A.; Martinez-Calcerrada, J.M.; Platero-Gutierrez, E.; De Zegher, F.; et al. Altered Circulating miRNA Expression Profile in Pregestational and Gestational Obesity. J. Clin. Endocrinol. Metab. 2015, 100, E1446–E1456. [Google Scholar] [CrossRef] [Green Version]
- Farr, R.J.; Januszewski, A.S.; Joglekar, M.V.; Liang, H.; McAulley, A.K.; Hewitt, A.W.; Thomas, H.E.; Loudovaris, T.; Kay, T.W.; Jenkins, A.; et al. A comparative analysis of high-throughput platforms for validation of a circulating microRNA signature in diabetic retinopathy. Sci. Rep. 2015, 5, 10375. [Google Scholar] [CrossRef] [Green Version]
- Blum, A.; Meerson, A.; Rohana, H.; Jabaly, H.; Nahul, N.; Celesh, D.; Romanenko, O.; Tamir, S. MicroRNA-423 may regulate diabetic vasculopathy. Clin. Exp. Med. 2019, 19, 469–477. [Google Scholar] [CrossRef]
- Ye, E.A.; Liu, L.; Jiang, Y.; Jan, J.; Gaddipati, S.; Suvas, S.; Steinle, J.J. miR-15a/16 reduces retinal leukostasis through decreased pro-inflammatory signaling. J. Neuroinflammation 2016, 13, 305. [Google Scholar] [CrossRef] [Green Version]
- Besnier, M.; Shantikumar, S.; Anwar, M.; Dixit, P.; Chamorro-Jorganes, A.; Sweaad, W.; Sala-Newby, G.; Madeddu, P.; Thomas, A.C.; Howard, L.; et al. miR-15a/-16 Inhibit Angiogenesis by Targeting the Tie2 Coding Sequence: Therapeutic Potential of a miR-15a/16 Decoy System in Limb Ischemia. Mol. Ther. Nucleic Acids 2019, 17, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Navitskaya, S.; Chakravarthy, H.; Huang, C.; Kady, N.; Lydic, T.A.; Chen, Y.E.; Yin, K.J.; Powell, F.L.; Martin, P.M.; et al. Dual Anti-Inflammatory and Anti-Angiogenic Action of miR-15a in Diabetic Retinopathy. EBioMedicine 2016, 11, 138–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, E.A.; Steinle, J.J. miR-15b/16 protects primary human retinal microvascular endothelial cells against hyperglycemia-induced increases in tumor necrosis factor alpha and suppressor of cytokine signaling 3. J. Neuroinflammation 2015, 12, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Dixon, B.J.; Doycheva, D.M.; Li, B.; Zhang, Y.; Hu, Q.; He, Y.; Guo, Z.; Nowrangi, D.; Flores, J.; et al. IRE1alpha inhibition decreased TXNIP/NLRP3 inflammasome activation through miR-17-5p after neonatal hypoxic-ischemic brain injury in rats. J. Neuroinflammation 2018, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.R.; Xue, W.L.; Zhang, L. Schisandrin B Attenuates Inflammation in LPS-Induced Sepsis Through miR-17-5p Downregulating TLR4. Inflammation 2019, 42, 731–739. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, L.; Li, Y.H.; Wang, Y.L.; Li, L. MiR-20a ameliorates diabetic angiopathy in streptozotocin-induced diabetic rats by regulating intracellular antioxidant enzymes and VEGF. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1948–1955. [Google Scholar]
- Bao, L.; Fu, X.; Si, M.; Wang, Y.; Ma, R.; Ren, X.; Lv, H. MicroRNA-185 targets SOCS3 to inhibit beta-cell dysfunction in diabetes. PLoS ONE 2015, 10, e0116067. [Google Scholar] [CrossRef]
- Hou, J.; Liu, L.; Zhu, Q.; Wu, Y.; Tian, B.; Cui, L.; Liu, Y.; Li, X. MicroRNA-185 inhibits angiogenesis in human microvascular endothelial cells through targeting stromal interaction molecule 1. Cell Biol. Int. 2016, 40, 318–328. [Google Scholar] [CrossRef]
- Qin, L.L.; An, M.X.; Liu, Y.L.; Xu, H.C.; Lu, Z.Q. MicroRNA-126: A promising novel biomarker in peripheral blood for diabetic retinopathy. Int. J. Ophthalmol. 2017, 10, 530–534. [Google Scholar]
- Fang, S.; Ma, X.; Guo, S.; Lu, J. MicroRNA-126 inhibits cell viability and invasion in a diabetic retinopathy model via targeting IRS-1. Oncol. Lett. 2017, 14, 4311–4318. [Google Scholar] [CrossRef]
- Mazzeo, A.; Beltramo, E.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp. Eye Res. 2018, 176, 69–77. [Google Scholar] [CrossRef]
- Ng, P.C.; Chan, K.Y.; Leung, K.T.; Tam, Y.H.; Ma, T.P.; Lam, H.S.; Cheung, H.M.; Lee, K.H.; To, K.F.; Li, K. Comparative MiRNA Expressional Profiles and Molecular Networks in Human Small Bowel Tissues of Necrotizing Enterocolitis and Spontaneous Intestinal Perforation. PLoS ONE 2015, 10, e0135737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snowhite, I.V.; Allende, G.; Sosenko, J.; Pastori, R.L.; Messinger Cayetano, S.; Pugliese, A. Association of serum microRNAs with islet autoimmunity, disease progression and metabolic impairment in relatives at risk of type 1 diabetes. Diabetologia 2017, 60, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Zheng, M.; Hayashi, M.; Lee, J.D.; Yoshino, O.; Lin, S.; Han, J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J. Clin. Investig. 2008, 118, 1944–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Yang, X.; Xie, B.; Chen, Y.; Swaim, M.; Hackett, S.F.; Campochiaro, P.A. MicroRNAs regulate ocular neovascularization. Mol. Ther. 2008, 16, 1208–1216. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Qiu, F.; Zhou, K.; Matlock, H.G.; Takahashi, Y.; Rajala, R.V.S.; Yang, Y.; Moran, E.; Ma, J.X. Pathogenic Role of microRNA-21 in Diabetic Retinopathy Through Downregulation of PPARalpha. Diabetes 2017, 66, 1671–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayyad-Kazan, H.; Bitar, N.; Najar, M.; Lewalle, P.; Fayyad-Kazan, M.; Badran, R.; Hamade, E.; Daher, A.; Hussein, N.; ElDirani, R.; et al. Circulating miR-150 and miR-342 in plasma are novel potential biomarkers for acute myeloid leukemia. J. Transl. Med. 2013, 11, 31. [Google Scholar] [CrossRef] [Green Version]
- Li, X.R.; Chu, H.J.; Lv, T.; Wang, L.; Kong, S.F.; Dai, S.Z. miR-342-3p suppresses proliferation, migration and invasion by targeting FOXM1 in human cervical cancer. FEBS Lett. 2014, 588, 3298–3307. [Google Scholar] [CrossRef] [Green Version]
- Estrella, S.; Garcia-Diaz, D.F.; Codner, E.; Camacho-Guillen, P.; Perez-Bravo, F. [Expression of miR-22 and miR-150 in type 1 diabetes mellitus: Possible relationship with autoimmunity and clinical characteristics]. Med. Clin. (Barc) 2016, 147, 245–247. [Google Scholar] [CrossRef]
- Mazzeo, A.; Lopatina, T.; Gai, C.; Trento, M.; Porta, M.; Beltramo, E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp. Eye Res. 2019, 184, 56–63. [Google Scholar] [CrossRef]
- Mansour, S.E.; Browning, D.J.; Wong, K.; Flynn, H.W., Jr.; Bhavsar, A.R. The Evolving Treatment of Diabetic Retinopathy. Clin. Ophthalmol. 2020, 14, 653–678. [Google Scholar] [CrossRef] [Green Version]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Rossino, M.G.; Casini, G. Nutraceuticals for the Treatment of Diabetic Retinopathy. Nutrients 2019, 11, 771. [Google Scholar] [CrossRef] [Green Version]
- Stewart, M.W. Corticosteroid use for diabetic macular edema: Old fad or new trend? Curr. Diab. Rep. 2012, 12, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Friedrichs, U.; Eichler, W.; Hoffmann, S.; Wiedemann, P. Inhibitory effects of triamcinolone acetonide on bFGF-induced migration and tube formation in choroidal microvascular endothelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, R.; Cicinelli, M.V.; Bandello, F. Intravitreal Steroids in Diabetic Macular Edema. Dev. Ophthalmol. 2017, 60, 78–90. [Google Scholar] [PubMed] [Green Version]
- Penfold, P.L.; Wen, L.; Madigan, M.C.; King, N.J.; Provis, J.M. Modulation of permeability and adhesion molecule expression by human choroidal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3125–3130. [Google Scholar]
- Penfold, P.L.; Wen, L.; Madigan, M.C.; Gillies, M.C.; King, N.J.; Provis, J.M. Triamcinolone acetonide modulates permeability and intercellular adhesion molecule-1 (ICAM-1) expression of the ECV304 cell line: Implications for macular degeneration. Clin. Exp. Immunol. 2000, 121, 458–465. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Zheng, Y. Glucocorticoids inhibit production of exosomes containing inflammatory microRNA-155 in lipopolysaccharide-induced macrophage inflammatory responses. Int. J. Clin. Exp. Pathol. 2018, 11, 3391–3397. [Google Scholar]
- Edelman, J.L.; Lutz, D.; Castro, M.R. Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown. Exp. Eye Res. 2005, 80, 249–258. [Google Scholar] [CrossRef]
- Lassota, N. Clinical and histological aspects of CNV formation: Studies in an animal model. Acta Ophthalmol. 2008, 86, 1–28. [Google Scholar] [CrossRef]
- Bhisitkul, R.B.; Winn, B.J.; Lee, O.T.; Wong, J.; Pereira Dde, S.; Porco, T.C.; He, X.; Hahn, P.; Dunaief, J.L. Neuroprotective effect of intravitreal triamcinolone acetonide against photoreceptor apoptosis in a rabbit model of subretinal hemorrhage. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Ayalasomayajula, S.P.; Amrite, A.C.; Kompella, U.B. Inhibition of cyclooxygenase-2, but not cyclooxygenase-1, reduces prostaglandin E2 secretion from diabetic rat retinas. Eur. J. Pharmacol. 2004, 498, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, H.; Rojas, M.; Caldwell, R.W.; Caldwell, R.B. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy 2011, 3, 609–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Gerhardinger, C.; Dagher, Z.; Hoehn, T.; Lorenzi, M. Aspirin at low-intermediate concentrations protects retinal vessels in experimental diabetic retinopathy through non-platelet-mediated effects. Diabetes 2005, 54, 3418–3426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, T.S.; Engerman, R.L. Pharmacological inhibition of diabetic retinopathy: Aminoguanidine and aspirin. Diabetes 2001, 50, 1636–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Group, T.D.S. Effect of aspirin alone and aspirin plus dipyridamole in early diabetic retinopathy. A multicenter randomized controlled clinical trial. The DAMAD Study Group. Diabetes 1989, 38, 491–498. [Google Scholar] [CrossRef]
- Hattori, Y.; Hashizume, K.; Nakajima, K.; Nishimura, Y.; Naka, M.; Miyanaga, K. The effect of long-term treatment with sulindac on the progression of diabetic retinopathy. Curr. Med. Res. Opin. 2007, 23, 1913–1917. [Google Scholar] [CrossRef]
- Amrite, A.C.; Ayalasomayajula, S.P.; Cheruvu, N.P.; Kompella, U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Kern, T.S.; Miller, C.M.; Du, Y.; Zheng, L.; Mohr, S.; Ball, S.L.; Kim, M.; Jamison, J.A.; Bingaman, D.P. Topical administration of nepafenac inhibits diabetes-induced retinal microvascular disease and underlying abnormalities of retinal metabolism and physiology. Diabetes 2007, 56, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Tsilimbaris, M.K.; Panagiotoglou, T.D.; Charisis, S.K.; Anastasakis, A.; Krikonis, T.S.; Christodoulakis, E. The use of intravitreal etanercept in diabetic macular oedema. Semin. Ophthalmol. 2007, 22, 75–79. [Google Scholar] [CrossRef]
- Markomichelakis, N.N.; Theodossiadis, P.G.; Sfikakis, P.P. Regression of neovascular age-related macular degeneration following infliximab therapy. Am. J. Ophthalmol. 2005, 139, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Suhler, E.B.; Smith, J.R.; Wertheim, M.S.; Lauer, A.K.; Kurz, D.E.; Pickard, T.D.; Rosenbaum, J.T. A prospective trial of infliximab therapy for refractory uveitis: Preliminary safety and efficacy outcomes. Arch. Ophthalmol. 2005, 123, 903–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, U.; Teml, A.; Schwab, M. Clinical pharmacokinetics and use of infliximab. Clin. Pharm. 2007, 46, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.S.; Zheng, Y.Z.; Huang, L.B.; Xu, G.X. Infliximab relieves blood retinal barrier breakdown through the p38 MAPK pathway in a diabetic rat model. Int. J. Ophthalmol. 2017, 10, 1824–1829. [Google Scholar] [PubMed]
- Stahel, M.; Becker, M.; Graf, N.; Michels, S. SYSTEMIC INTERLEUKIN 1beta INHIBITION IN PROLIFERATIVE DIABETIC RETINOPATHY: A Prospective Open-Label Study Using Canakinumab. Retina 2016, 36, 385–391. [Google Scholar] [CrossRef] [Green Version]
- Sjolie, A.K.; Dodson, P.; Hobbs, F.R. Does renin-angiotensin system blockade have a role in preventing diabetic retinopathy? A clinical review. Int. J. Clin. Pract. 2011, 65, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Tamsma, J.T. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 2009, 361, 1410. [Google Scholar]
- Chaturvedi, N.; Porta, M.; Klein, R.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Sjolie, A.K.; DIRECT Programme Study Group. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: Randomised, placebo-controlled trials. Lancet 2008, 372, 1394–1402. [Google Scholar] [CrossRef]
- Sjolie, A.K.; Klein, R.; Porta, M.; Orchard, T.; Fuller, J.; Parving, H.H.; Bilous, R.; Chaturvedi, N.; DIRECT Programme Study Group. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): A randomised placebo-controlled trial. Lancet 2008, 372, 1385–1393. [Google Scholar] [CrossRef]
- Haque, R.; Hur, E.H.; Farrell, A.N.; Iuvone, P.M.; Howell, J.C. MicroRNA-152 represses VEGF and TGFbeta1 expressions through post-transcriptional inhibition of (Pro)renin receptor in human retinal endothelial cells. Mol. Vis. 2015, 21, 224–235. [Google Scholar]
- Goncalves, A.; Marques, C.; Leal, E.; Ribeiro, C.F.; Reis, F.; Ambrosio, A.F.; Fernandes, R. Dipeptidyl peptidase-IV inhibition prevents blood-retinal barrier breakdown, inflammation and neuronal cell death in the retina of type 1 diabetic rats. Biochim. Biophys. Acta 2014, 1842, 1454–1463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, A.; Leal, E.; Paiva, A.; Teixeira Lemos, E.; Teixeira, F.; Ribeiro, C.F.; Reis, F.; Ambrosio, A.F.; Fernandes, R. Protective effects of the dipeptidyl peptidase IV inhibitor sitagliptin in the blood-retinal barrier in a type 2 diabetes animal model. Diabetes Obes. Metab. 2012, 14, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Almeida, L.; Silva, A.P.; Fontes-Ribeiro, C.; Ambrosio, A.F.; Cristovao, A.; Fernandes, R. The dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin ameliorates retinal endothelial cell dysfunction triggered by inflammation. Biomed. Pharmacother. 2018, 102, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, K.; Wang, F.; Zhou, L.; Hu, Y.; Tang, M.; Zhang, S.; Jin, S.; Zhang, J.; Wang, J.; et al. The glucagon like peptide 1 analogue, exendin-4, attenuates oxidative stress-induced retinal cell death in early diabetic rats through promoting Sirt1 and Sirt3 expression. Exp. Eye Res. 2016, 151, 203–211. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, K.; Wang, Q.; Ruan, Y.; Ye, W.; Zhang, Y. Exendin-4 alleviates retinal vascular leakage by protecting the blood-retinal barrier and reducing retinal vascular permeability in diabetic Goto-Kakizaki rats. Exp. Eye Res. 2014, 127, 104–116. [Google Scholar] [CrossRef]
- Hernandez, C.; Bogdanov, P.; Corraliza, L.; Garcia-Ramirez, M.; Sola-Adell, C.; Arranz, J.A.; Arroba, A.I.; Valverde, A.M.; Simo, R. Topical Administration of GLP-1 Receptor Agonists Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 2016, 65, 172–187. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M. A glucagon-like peptide-1 analog, liraglutide, ameliorates endothelial dysfunction through miRNAs to inhibit apoptosis in rats. PeerJ 2019, 7, e6567. [Google Scholar] [CrossRef] [Green Version]
- Al-Janabi, A.; Lightman, S.; Tomkins-Netzer, O. ‘Statins in retinal disease’. Eye (Lond.) 2018, 32, 981–991. [Google Scholar] [CrossRef]
- Yeh, P.T.; Wang, L.C.; Chang, S.W.; Yang, W.S.; Yang, C.M.; Yang, C.H. Effect of Fenofibrate on the Expression of Inflammatory Mediators in a Diabetic Rat Model. Curr. Eye Res. 2019, 44, 1121–1132. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Tang, J.; Kern, T.S. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII. Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes 2001, 50, 1938–1942. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.K.; El-Remessy, A.B. Diabetic retinopathy: Current management and experimental therapeutic targets. Pharmacotherapy 2009, 29, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.J.; Xiong, S.Q.; Ding, L.X.; Peng, J.; Xia, X.B. Diabetic retinopathy: Focus on NADPH oxidase and its potential as therapeutic target. Eur. J. Pharmacol. 2019, 853, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabrawey, M.; Rojas, M.; Sanders, T.; Behzadian, A.; El-Remessy, A.; Bartoli, M.; Parpia, A.K.; Liou, G.; Caldwell, R.B. Role of NADPH oxidase in retinal vascular inflammation. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3239–3244. [Google Scholar] [CrossRef]
- Zhang, W.; Rojas, M.; Lilly, B.; Tsai, N.T.; Lemtalsi, T.; Liou, G.I.; Caldwell, R.W.; Caldwell, R.B. NAD(P)H oxidase-dependent regulation of CCL2 production during retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Koppolu, P.; Chakrabarti, S.; Chen, S. Diabetes-induced activation of nuclear transcriptional factor in the retina, and its inhibition by antioxidants. Free Radic. Res. 2003, 37, 1169–1180. [Google Scholar] [CrossRef]
- Diabetes: Oxidative Stress and Dietary Antioxidants, 2nd ed.; Preedy, V.R. (Ed.) Elsevier: Amsterdam, The Netherlands, 2020; 454p. [Google Scholar]
- Moisseiev, E.; Anderson, J.D.; Oltjen, S.; Goswami, M.; Zawadzki, R.J.; Nolta, J.A.; Park, S.S. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr. Eye Res. 2017, 42, 1358–1367. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, 34562. [Google Scholar] [CrossRef] [Green Version]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.H.; Shamardan, R.M. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes Derived from Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Investig. Ophthalmol. Vis. Sci. 2019, 60, 294–303. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, B.; Amorim, M.; Reis, F.; Ambrósio, A.F.; Fernandes, R. Extracellular Vesicles and MicroRNA: Putative Role in Diagnosis and Treatment of Diabetic Retinopathy. Antioxidants 2020, 9, 705. https://doi.org/10.3390/antiox9080705
Martins B, Amorim M, Reis F, Ambrósio AF, Fernandes R. Extracellular Vesicles and MicroRNA: Putative Role in Diagnosis and Treatment of Diabetic Retinopathy. Antioxidants. 2020; 9(8):705. https://doi.org/10.3390/antiox9080705
Chicago/Turabian StyleMartins, Beatriz, Madania Amorim, Flávio Reis, António Francisco Ambrósio, and Rosa Fernandes. 2020. "Extracellular Vesicles and MicroRNA: Putative Role in Diagnosis and Treatment of Diabetic Retinopathy" Antioxidants 9, no. 8: 705. https://doi.org/10.3390/antiox9080705