New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes
Abstract
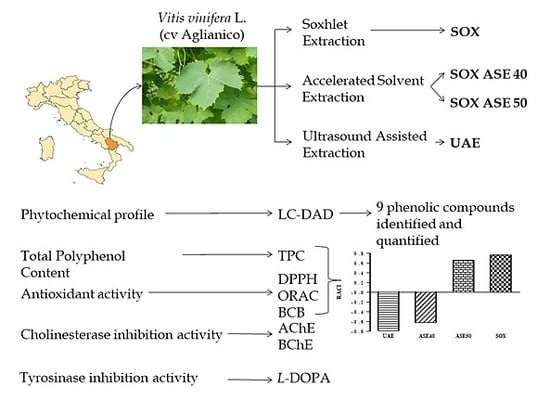
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Grape Leaves Collection
2.3. Extraction of Antioxidant Compounds
2.3.1. Soxhlet Extraction (SOX)
2.3.2. Accelerated Solvent Extraction (ASE)
2.3.3. Ultrasound Assisted Extraction (UAE)
2.4. RP-HPLC-DAD Qualitative and Quantitative Analysis of Phenolic Compounds
2.5. Total Phenolic Content (TPC)
2.6. Antioxidant Activity
2.6.1. DPPH Method
2.6.2. Oxygen Radical Antioxidant Capacity (ORAC) assay
2.6.3. β-Carotene Bleaching Assay (BCB)
2.7. Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Inhibitory Activity
2.8. Tyrosinase Inhibitory Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Vitis vinifera cv. Aglianico Leaf Extraction
3.2. Identification and Quantification of Phenolic Compounds
3.3. Total Polyphenol Content and Antioxidant Activity
3.4. Inhibitory Activity Against Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE)
3.5. Inhibitory Activity Against Tyrosinase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 March 2020).
- Gabaston, J.; El Khawand, T.; Waffo-Teguo, P.; Decendit, A.; Richard, T.; Mérillon, J.-M.; Pavela, R. Stilbenes from grapevine root: A promising natural insecticide against Leptinotarsa decemlineata. J. Pest. Sci. 2018, 91, 897–906. [Google Scholar] [CrossRef]
- Dukić, D.; Mašković, P.; Moračanin, S.V.; Kurćubić, V.; Milijašević, M.; Babić, J. Conventional and unconventional extraction methods applied to the plant, Thymus serpyllum L. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Zlatibor, Serbia, 1–4 October 2017. [Google Scholar]
- Russo, D.; Faraone, I.; Labanca, F.; Sinisgalli, C.; Bartolo, M.; Andrade, P.B.; Valentao, P.; Milella, L. Comparison of different green-extraction techniques and determination of the phytochemical profile and antioxidant activity of Echinacea angustifolia L. extracts. Phytochem. Anal. 2019, 30, 547–555. [Google Scholar] [CrossRef] [PubMed]
- De la Cerda-Carrasco, A.; López-Solís, R.; Nuñez-Kalasic, H.; Peña-Neira, Á.; Obreque-Slier, E. Phenolic composition and antioxidant capacity of pomaces from four grape varieties (Vitis vinifera L.). J. Sci. Food Agric. 2015, 95, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Cafaro, C.; Bonomo, M.G.; Guerrieri, A.; Crispo, F.; Ciriello, R.; Salzano, G. Assessment of the genetic polymorphism and physiological characterization of indigenous Oenococcus oeni strains isolated from Aglianico del Vulture red wine. Folia Microbiol. 2016, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Nisco, M.; Manfra, M.; Bolognese, A.; Sofo, A.; Scopa, A.; Tenore, G.C.; Pagano, F.; Milite, C.; Russo, M.T. Nutraceutical properties and polyphenolic profile of berry skin and wine of Vitis vinifera L. (cv. Aglianico). Food Chem. 2013, 140, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Jourdes, M.; Teissedre, P.; Moio, L. A preliminary characterization of Aglianico (Vitis vinifera L. cv.) grape proanthocyanidins and evaluation of their reactivity towards salivary proteins. Food Chem. 2014, 164, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Villano, C.; Lanzillo, C.; Tamburrino, A., Jr.; Jourdes, M.; Teissedre, P.-L.; Moio, L.; Frusciante, L.; Carputo, D.; Aversano, R. Metabolic and RNA profiling elucidates proanthocyanidins accumulation in Aglianico grape. Food Chem. 2017, 233, 52–59. [Google Scholar] [CrossRef]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriele, M.; Gerardi, C.; Lucejko, J.J.; Longo, V.; Pucci, L.; Domenici, V. Effects of low sulfur dioxide concentrations on bioactive compounds and antioxidant properties of Aglianico red wine. Food Chem. 2018, 245, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, L.; Binello, A.; Mantegna, S.; Boffa, L.; Chemat, F.; Cravotto, G. Efficient green extraction of polyphenols from post-harvested agro-industry vegetal sources in Piedmont. C. R. Chim. 2014, 17, 212–217. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. In Vitro 2010, 24, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.R.; Felgueiras, M.L.; Cunha, A.; Chicau, G.; Ferreres, F.; Dias, A.C. Differential phenolic production in leaves of Vitis vinifera cv. Alvarinho affected with esca disease. Plant Physiol. Biochem. 2017, 112, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Crupi, P.; Dipalmo, T.; Clodoveo, M.L.; Toci, A.T.; Coletta, A. Seedless table grape residues as a source of polyphenols: Comparison and optimization of non-conventional extraction techniques. Eur. Food Res. Technol. 2018, 244, 1091–1100. [Google Scholar] [CrossRef]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Katalinić, V.; Generalić, I.; Skroza, D.; Ljubenkov, I.; Teskera, A.; Konta, I.; Boban, M. Insight in the phenolic composition and antioxidative properties of Vitis vinifera leaves extracts. Croat. J. Food Sci. Technol. 2009, 1, 7–15. [Google Scholar]
- Ferhi, S.; Santaniello, S.; Zerizer, S.; Cruciani, S.; Fadda, A.; Sanna, D.; Dore, A.; Maioli, M.; D’hallewin, G. Total phenols from grape leaves counteract cell proliferation and modulate apoptosis-related gene expression in MCF-7 and HepG2 human cancer cell lines. Molecules 2019, 24, 612. [Google Scholar] [CrossRef] [Green Version]
- Neagu, E.; Radu, G.L.; Albu, C.; Paun, G. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J. Biol. Sci. 2018, 25, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Greggio, E.; Bergantino, E.; Carter, D.; Ahmad, R.; Costin, G.E.; Hearing, V.J.; Clarimon, J.; Singleton, A.; Eerola, J.; Hellström, O. Tyrosinase exacerbates dopamine toxicity but is not genetically associated with Parkinson’s disease. J. Neurochem. 2005, 93, 246–256. [Google Scholar] [CrossRef]
- Eruygur, N.; Koçyiğit, U.; Taslimi, P.; Ataş, M.; Tekin, M.; Gülçin, İ. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef]
- Rizk, M.; Borai, I.; Ezz, M.; El-Sherbiny, M.; Aly, H.; Matloub, A.; Fouad, G. Possible therapeutic role of grape (Vitis vinifera) leaves polyphenolic extract in the regression of aluminium-induced Alzheimer’s disease in rats. J. Mater. Environ. Sci. 2018, 9, 2098–2108. [Google Scholar]
- De Castro, M.L.; Garcıa-Ayuso, L. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Herrero, M.; Ibáñez, E.; Señoráns, J.; Cifuentes, A. Pressurized liquid extracts from Spirulina platensis microalga: Determination of their antioxidant activity and preliminary analysis by micellar electrokinetic chromatography. J. Chromatogr. A 2004, 1047, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Todaro, L.; Russo, D.; Cetera, P.; Milella, L. Effects of thermo-vacuum treatment on secondary metabolite content and antioxidant activity of poplar (Populus nigra L.) wood extracts. Ind. Crops Prod. 2017, 109, 384–390. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Chen, H.-J.; Huang, J.-P.; Lee, P.-C.; Tsai, C.-R.; Hsu, T.-F.; Huang, W.-Y. Kinetics of tyrosinase inhibitory activity using Vitis vinifera leaf extracts. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Betés-Saura, C.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Phenolics in white free run juices and wines from Penedès by high-performance liquid chromatography: Changes during vinification. J. Agric. Food Chem. 1996, 44, 3040–3046. [Google Scholar] [CrossRef]
- Fernández, E.C.; Rajchl, A.; Lachman, J.; Čížková, H.; Kvasnička, F.; Kotíková, Z.; Milella, L.; Voldřich, M. Impact of yacon landraces cultivated in the Czech Republic and their ploidy on the short-and long-chain fructooligosaccharides content in tuberous roots. LWT Food Sci. Technol. 2013, 54, 80–86. [Google Scholar] [CrossRef]
- Milella, L.; Caruso, M.; Galgano, F.; Favati, F.; Padula, M.C.; Martelli, G. Role of the cultivar in choosing Clementine fruits with a high level of health-promoting compounds. J. Agric. Food Chem. 2011, 59, 5293–5298. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Krisa, S.; García-Parrilla, M.C.; Richard, T. Effects of gluconic and alcoholic fermentation on anthocyanin composition and antioxidant activity of beverages made from strawberry. LWT Food Sci. Technol. 2016, 69, 382–389. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Cuevas, E.; Winterhalter, P.; Garcia-Parrilla, M.; Troncoso, A. Isolation, identification, and antioxidant activity of anthocyanin compounds in Camarosa strawberry. Food Chem. 2010, 123, 574–582. [Google Scholar] [CrossRef]
- Russo, D.; Valentão, P.; Andrade, P.; Fernandez, E.; Milella, L. Evaluation of antioxidant, antidiabetic and anticholinesterase activities of Smallanthus sonchifolius landraces and correlation with their phytochemical profiles. Int. J. Mol. Sci. 2015, 16, 17696–17718. [Google Scholar] [CrossRef] [Green Version]
- Milella, L.; Milazzo, S.; De Leo, M.; Vera Saltos, M.B.; Faraone, I.; Tuccinardi, T.; Lapillo, M.; De Tommasi, N.; Braca, A. α-Glucosidase and α-amylase inhibitors from Arcytophyllum thymifolium. J. Nat. Prod. 2016, 79, 2104–2112. [Google Scholar] [CrossRef]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complementary Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef] [Green Version]
- Saltos, M.B.V.; Puente, B.F.N.; Faraone, I.; Milella, L.; De Tommasi, N.; Braca, A. Inhibitors of α-amylase and α-glucosidase from Andromachia igniaria Humb. & Bonpl. Phytochem. Lett. 2015, 14, 45–50. [Google Scholar]
- Sun, T.; Tanumihardjo, S. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef]
- Silla, E.; Arnau, A.; Tunon, I. Solvent Effects on Chemical Systems; ChemTec Publishing: Toronto, NY, USA, 2001; pp. 7–63. [Google Scholar]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matloub, A.A. Optimization of polyphenol extraction from Vitis vinifera L. leaves, antioxidant activity and its correlation with amelioration effect on AlCl3-induced Alzheimer’s disease. Arch. Pharm. Sciences Ain Shams Univ. 2018, 2, 97–110. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Aouey, B.; Samet, A.M.; Fetoui, H.; Simmonds, M.S.; Bouaziz, M. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC–MS/MS analyses. Biomed. Pharmacother. 2016, 84, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, T.; Loyola, C.; de Bruijn, J.; Bustamante, L.; Vergara, C.; von Baer, D.; Mardones, C.; Serra, I. Effect of thermomaceration and enzymatic maceration on phenolic compounds of grape must enriched by grape pomace, vine leaves and canes. Eur. Food Res. Technol. 2016, 242, 1149–1158. [Google Scholar] [CrossRef]
- Amarowicz, R.; Narolewska, O.; Karamac, M.; Kosinska, A.; Weidner, S. Grapevine leaves as a source of natural antioxidants. Pol. J. Food Nutr. Sci. 2008, 58, 73–78. [Google Scholar]
- Guidoni, S.; Mannini, F.; Ferrandino, A.; Argamante, N.; Di Stefano, R. The effect of grapevine leafroll and rugose wood sanitation on agronomic performance and berry and leaf phenolic content of a Nebbiolo clone (Vitis vinifera L.). Am. J. Enol. Vitic. 1997, 48, 438–442. [Google Scholar]
- Monagas, M.; Hernández-Ledesma, B.; Gómez-Cordovés, C.; Bartolomé, B. Commercial dietary ingredients from Vitis vinifera L. leaves and grape skins: Antioxidant and chemical characterization. J. Agric. Food Chem. 2006, 54, 319–327. [Google Scholar] [CrossRef]
- Weber, B. Phenolische komponenten des weinrebenblattes: Identität und phytopathologische Bedeutung. Ph.D. Thesis, University of Zürich, Zürich, Switzerland, 1992. [Google Scholar]
- Uysal, S.; Zengin, G.; Aktumsek, A.; Karatas, S. Chemical and biological approaches on nine fruit tree leaves collected from the Mediterranean region of Turkey. J. Funct. Foods 2016, 22, 518–532. [Google Scholar] [CrossRef]
- Dekdouk, N.; Malafronte, N.; Russo, D.; Faraone, I.; De Tommasi, N.; Ameddah, S.; Severino, L.; Milella, L. Phenolic compounds from Olea europaea L. possess antioxidant activity and inhibit carbohydrate metabolizing enzymes in vitro. Evid. Based Complement. Alternat. Med. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Giacobini, E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol. Res. 2004, 50, 433–440. [Google Scholar] [CrossRef]
- Orhan, I.E.; Akkol, E.K.; Suntar, I.; Yesilada, E. Assessment of anticholinesterase and antioxidant properties of the extracts and (+)-catechin obtained from Arceuthobium oxycedri (DC) M. Bieb (dwarf mistletoe). S. Afr. J. Bot. 2019, 120, 309–312. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. CMLS 2005, 62, 1707–1723. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, A.; Teruel-Puche, J.A.; Garcia-Ruiz, P.A.; Saura-Sanmartin, A.; Berna, J.; Rodríguez-López, J.N.; Garcia-Canovas, F. Action of tyrosinase on caffeic acid and its n-nonyl ester. Catalysis and suicide inactivation. Int. J. Biol. Macromol. 2018, 107, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Honisch, C.; Osto, A.; de Matos, A.D.; Vincenzi, S.; Ruzza, P. Isolation of a tyrosinase inhibitor from unripe grapes juice: A spectrophotometric study. Food Chem. 2020, 305, 125506. [Google Scholar] [CrossRef]
Data Availability: All dataset used for this study is available on request. |



| Method of Extraction | Solvent of Extraction | G | Temperature | Time of Extraction | Extraction Yield (%) |
|---|---|---|---|---|---|
| Soxhlet extraction (SOX) | Ultrapure water and ethanol, 50:50 | 25.40 g | 100 °C | 6 h | 30.45 ± 2.32 b |
| Ultrasound Assisted Extraction (UAE) | Ultrapure water and ethanol, 50:50 | 10.27 g | 30 °C | 6 h | 13.81 ± 1.13 c |
| Accelerated Solvent Extraction (ASE 40) | Ultrapure water and ethanol, 50:50 | 30.29 g | 40 °C | static time 5 min × 3 cycles | 6.44 ± 0.48 a |
| Accelerated Solvent Extraction (ASE 50) | Ultrapure water and ethanol, 50:50 | 26.17 g | 50 °C | static time 5 min × 3 cycles | 6.41 ± 0.52 a |
| Peak | Analyte | RT (min) | λmax | SOX | ASE 40 | ASE 50 | UAE |
|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 3.9 | 280 | 159.91 ± 1.54 a | Nd | Nd | Nd |
| 2 | Caftaric acid | 6.7 | 320 | 5706.97 ± 77.20 a | 3651.71 ± 52.91 b | 4075.15 ± 23.40 c | 6047.84 ± 41.30 d |
| 3 | (+)-Catechin | 14.7 | 280 | 1176.00 ± 17.32 a | 300.52 ± 0.41 b | 331.72 ± 0.52 c | 682.81 ± 2.72 d |
| 4 | Caffeic acid | 16.3 | 320 | 557.31 ± 2.68 a | Nd | Nd | Nd |
| 5 | Benzoic acid | 21.8 | 280 | 131.23 ± 4.89 a | 452.83 ± 2.76 b | 315.78 ± 1.85 c | 408.27 ± 0.00 d |
| 6 | Rutin | 28.1 | 365 | 205.69 ± 15.34 a | 210.64 ± 7.20 a | 192.23 ± 18.89 a | 319.75 ± 1.49 b |
| 7 | Quercetin-3-O-galactoside | 29.5 | 365 | 2938.56 ± 6.79 a | 1976.24 ± 20.78 b | 2003.21 ± 16.80 b | 2449.91 ± 3.43 c |
| 8 | Quercetin-3-O-glucoside | 29.9 | 365 | 2352.24 ± 41.34 a | Nd | Nd | Nd |
| 9 | Quercetin -3-O-glucuronide | 30.3 | 365 | 2893.19 ± 21.59 a | 2009.87 ± 5.99 b | 2023.01 ± 3.45 b | 2465.27 ± 3.43 c |
| 10 | Quercetin-3-O-glycoside | 30.8 | 365 | 3362.17 ± 4.94 a | 2219.28 ± 1.60 b | 2450.58 ± 23.26 c | 3150.82 ± 10.29 d |
| 11 | Kaempferol-3-O-glucoside | 33.6 | 365 | 666.64 ± 6.10 a | 562.06 ± 24.41 b | 535.27 ± 24.99 b | 572.65 ± 10.39 b |
| 12 | Quercetin | 38.1 | 365 | 490.71 ± 0.63 a | Nd | Nd | Nd |
| TOTAL | 20640.62 ± 200.36 | 11383.15 ± 116.06 | 11926.95 ± 96.36 | 16097.32 ± 73.05 |
| Samples | % Inhibition (at 125 μg/mL) | IC50 (µg/mL) * | ||
|---|---|---|---|---|
| AChE | BChE | AChE | BChE | |
| Galantamine | 95.82 ± 1.60 | 97.55 ± 1.60 | 1.53 ± 0.11 | 1.85 ± 0.08 |
| SOX | 31.29 ± 0.09 a | 43.85 ± 2.17 a | Nd | 171.34 ± 12.12 |
| ASE 40 | 34.60 ± 2.05 a | 4.19 ± 0.35 b,c | Nd | Nd |
| ASE 50 | 50.65 ± 3.12 b | 15.65 ±0.21 b,d | 107.16 ± 8.12 | Nd |
| UAE | 25.52 ± 0.61 a | 11.8 ± 1.6 c,d | Nd | Nd |
| Samples | IC50 (µg/mL) * |
|---|---|
| Tyrosinase | |
| Kojic acid | 3.9 ± 0.49 a |
| SOX | 302.5 ± 38.30 b |
| ASE 40 | 568.7 ± 1.801 c |
| ASE 50 | 727.1 ± 48.23 d |
| UAE | 293.2 ± 25.60 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes. Antioxidants 2020, 9, 708. https://doi.org/10.3390/antiox9080708
Labanca F, Faraone I, Nolè MR, Hornedo-Ortega R, Russo D, García-Parrilla MC, Chiummiento L, Bonomo MG, Milella L. New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes. Antioxidants. 2020; 9(8):708. https://doi.org/10.3390/antiox9080708
Chicago/Turabian StyleLabanca, Fabiana, Immacolata Faraone, Maria Rosaria Nolè, Ruth Hornedo-Ortega, Daniela Russo, Maria Carmen García-Parrilla, Lucia Chiummiento, Maria Grazia Bonomo, and Luigi Milella. 2020. "New Insights into the Exploitation of Vitis vinifera L. cv. Aglianico Leaf Extracts for Nutraceutical Purposes" Antioxidants 9, no. 8: 708. https://doi.org/10.3390/antiox9080708