Abstract
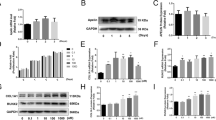
Local injection of tumor necrosis factor-alpha (TNF-α) at bone fracture sites during the early stage of the inflammatory response is reported to improve fracture repair in a murine model. However, the underlying mechanism is unclear. Endochondral bone formation, a process that is highly related to fracture repair, requires a certain amount of chondrocyte hypertrophy. This study aimed to investigate the effect of TNF-α on the differentiation of murine chondrogenic ATDC5 cells and the underlying mechanism. In this study, improved chondrogenic differentiation of ATDC5 cells was achieved by brief TNF-α stimulation. Moreover, the expression of Yes-associated protein 1 (YAP1) was suppressed after brief TNF-α stimulation. The expressions of inflammatory mediators and chondrogenic and hypertrophic-associated genes in ATDC5 cells triggered by TNF-α were suppressed in the YAP1 overexpression group but enhanced in the YAP1 knockdown group. Mechanistically, TNF-α-induced activation of the 5ʹ AMP-activated protein kinase (AMPK) signaling pathway was regulated by YAP1, as revealed by the phosphorylated-AMPK/AMPK change ratios in the YAP1 overexpression and knockdown groups, respectively. Moreover, the potential for TNF-α to enhance chondrogenic differentiation could be partially reversed with an AMPK inhibitor. Taken together, we demonstrate, for the first time, that YAP1 modulates the ability of TNF-α to enhance chondrocyte differentiation partly through AMPK signaling.




Similar content being viewed by others
Data availability
The datasets generated or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Baht GS, Vi L, Alman BA (2018) The role of the immune cells in fracture healing. Curr Osteoporos Rep 16:138–145. https://doi.org/10.1007/s11914-018-0423-2
Ai-Aql ZS, Alagl AS, Graves DT, Gerstenfeld LC, Einhorn TA (2008) Molecular mechanisms controlling bone formation during fracture healing and distraction osteogenesis. J Dent Res 87:107–118. https://doi.org/10.1177/154405910808700215
Gibon E, Lu L, Goodman SB (2016) Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther 22:7–44. https://doi.org/10.1186/s13287-016-0300-9
Osta B, Benedetti G, Miossec P (2014) Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol. https://doi.org/10.3389/fimmu.2014.00048
Lu Z, Wang G, Dunstan CR, Zreiqat H (2012) Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived MSC into osteogenic differentiation. Stem Cells Dev 21:2420–2429. https://doi.org/10.1186/s13287-016-0300-9
Daniele S, Natali L, Giacomelli C, Campiglia P, Novellino E, Martini C, Trincavelli ML (2017) Osteogenesis is improved by low tumor necrosis factor alpha concentration through the modulation of Gs-coupled receptor signals. Mol Cell Biol. https://doi.org/10.1128/MCB.00442-16
Chan JK, Glass GE, Ersek A, Freidin A, Williams GA, Gowers K, Espirito Santo AI, Jeffery R, Otto WR, Poulsom R, Feldmann M, Rankin SM, Horwood NJ, Nanchahal J (2015) Low-dose TNF-α augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol Med 7:547–561. https://doi.org/10.15252/emmm.201404487
Li J, Dong S (2016) The signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int. https://doi.org/10.1155/2016/2470351
Huang J, Wu S, Barrera J, Matthews K, Pan D (2005) The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the drosophila homolog of YAP. Cell 122:421–434. https://doi.org/10.1016/j.cell.2005.06.007
Yu F, Zhao B, Guan K (2015) Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163:811–828. https://doi.org/10.1016/j.cell.2015.10.044
Yang B, Sun H, Song F, Yu M, Wu Y, Wang J (2017) YAP1 negatively regulates chondrocyte differentiation partly by activating the beta-catenin signaling pathway. Int J Biochem Cell Biol 87:104–113. https://doi.org/10.1016/j.biocel.2017.04.007
Deng Y, Wu A, Li P, Li G, Qin L, Song H, Mak KK (2016) Yap1 regulates multiple steps of chondrocyte differentiation during skeletal development and bone repair. Cell Rep 14:2224–2237. https://doi.org/10.1016/j.celrep.2016.02.02
Zhang Q, Han X, Chen J, Xie X, Xu J, Zhao Y, Shen J, Hu L, Xu P, Song H, Zhang L, Zhao B, Wang YJ, Xia Z (2018) Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) mediate cell density–dependent proinflammatory responses. J Biol Chem 293:18071–18085. https://doi.org/10.1074/jbc.RA118.004251
Wang S, Xie F, Chu F, Zhang Z, Yang B, Dai T, Gao L, Wang L, Ling L, Jia J, van Dam H, Jin J, Zhang L, Zhou F (2017) YAP antagonizes innate antiviral immunity and is targeted for lysosomal degradation through IKKɛ-mediated phosphorylation. Nat Immunol 18:733–743. https://doi.org/10.1038/ni1117-1270d
Deng Y, Lu J, Li W, Wu A, Zhang X, Tong W, Ho KK, Qin L, Song H, Mak KK (2018) Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat Commun 9:4564. https://doi.org/10.1038/s41467-018-07022-2
Akutsu M, Ogura N, Ito K, Kawashima M, Kishida T, Kondoh T (2013) Effects of interleukin-1beta and tumor necrosis factor-alpha on macrophage inflammatory protein-3alpha production in synovial fibroblast-like cells from human temporomandibular joints. J Oral Pathol Med 42:491–498. https://doi.org/10.1111/jop.12040
Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ (2011) Structure of mammalian AMPK and its regulation by ADP. Nature 472:230–233. https://doi.org/10.1038/ncb3113
Li Y, Su J, Sun W, Cai L, Deng Z (2018) AMP-activated protein kinase stimulates osteoblast differentiation and mineralization through autophagy induction. Int J Mol Med 41:2535–2544. https://doi.org/10.3892/ijmm.2018.3498
Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J (2015) AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol 17:490–499. https://doi.org/10.1038/ncb3113
Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA (2003) Impaired fracture healing in the absence of TNF-α signaling: the role of TNF-α in endochondral cartilage resorption. J Bone Miner Res 18:1584–1592. https://doi.org/10.1359/jbmr.2003.18.9.1584
Siggelkow H, Eidner T, Lehmann G, Viereck V, Raddatz D, Munzel U, Hein G, Hüfner M (2003) Cytokines, osteoprotegerin, and RANKL in vitro and histomorphometric indices of bone turnover in patients with different bone diseases. J Bone Miner Res 18:529–538. https://doi.org/10.1359/jbmr.2003.18.3.529
Zhao B (2017) TNF-α and bone remodeling. Curr Osteoporos Rep 15:126–134. https://doi.org/10.1007/s11914-017-0358-z
Yao Y, Wang Y (2013) ATDC5: an excellent in vitro model cell line for skeletal development. J Cell Biochem 114:1223–1229. https://doi.org/10.1002/jcb.24467
Ke Y, Liu C, Wang Y, Xiao M, Fan J, Fu P, Wang S, Wu G (2018) Cell-loaded carboxymethylcellulose microspheres sustain viability and proliferation of ATDC5 cells. Artif Cells Nanomed Biotechnol 46(sup2):140–151. https://doi.org/10.1080/21691401.2018.1452751
Sumitani Y, Uchibe K, Yoshida K, Weng Y, Guo J, Yuan H, Ikegame M, Kamioka H, Okamura H (2019) Inhibitory effect of retinoic acid receptor agonists on in vitro chondrogenic differentiation. Anat Sci Int 95:202–208. https://doi.org/10.1007/s12565-019-00512-
Wakabayashi T, Matsumine A, Nakazora S, Hasegawa M, Iino T, Ota H, Sonoda H, Sudo A, Uchida A (2010) Fibulin-3 negatively regulates chondrocyte differentiation. Biochem Biophys Res Commun 391:1116–1121. https://doi.org/10.1016/j.bbrc.2009.12.034
Claes L, Recknagel S, Ignatius A (2012) Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 8:133–143. https://doi.org/10.1038/nrrheum.2012.1
Einhorn TA, Gerstenfeld LC (2014) Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 11:45–54. https://doi.org/10.1038/nrrheum.2014.164
Lefebvre V, de Crombrugghe B (1998) Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol 16:529–540. https://doi.org/10.1016/S0945-053X(98)90065-8
Kozhemyakina E, Lassar AB, Zelzer E (2015) A pathway to bone signaling molecules and transcription factors involved in chondrocyte development and maturation. Development 142:817–831. https://doi.org/10.1242/dev.105536
Yang X, Ricciardi BF, Hernandez-Soria A, Shi Y, Pleshko Camacho N, Bostrom MP (2007) Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 41:928–936. https://doi.org/10.1016/j.bone.2007.07.022
Baldik Y, Diwan AD, Appleyard RC, Fang ZM, Wang Y, Murrell GA (2005) Deletion of iNOS gene impairs mouse fracture healing. Bone 37:32–36. https://doi.org/10.1016/j.bone.2004.10.002
Kosaki N, Takaishi H, Kamekura S, Kimura T, Okada Y, Minqi L, Amizuka N, Chung UI, Nakamura K, Kawaguchi H, Toyama Y, D'Armiento J (2007) Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun 354:846–851. https://doi.org/10.1016/j.bbrc.2006.12.234
Janssen MP, Caron MM, van Rietbergen B, Surtel DA, van Rhijn LW, Welting TJ, Emans PJ (2017) Impairment of the chondrogenic phase of endochondral ossification in vivo by inhibition of cyclooxygenase-2. Eur Cell Mater 34:202–216. https://doi.org/10.22203/eCM.v034a13
Bai Y, Liu C, Fu L, Gong X, Dou C, Cao Z, Quan H, Li J, Kang F, Dai J, Zhao C, Dong S (2018) Mangiferin enhances endochondral ossification-based bone repair in massive bone defect by inducing autophagy through activating AMP-activated protein kinase signaling pathway. FASEB J 32:4573–4584. https://doi.org/10.1096/fj.201701411R
O'Neill LA, Hardie DG (2013) Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493:346–355. https://doi.org/10.1038/nature11862
Ding L, Yuan X, Yan J, Huang Y, Xu M, Yang Z, Yang N, Wang M, Zhang C, Zhang L (2019) Nrf2 exerts mixed inflammation and glucose metabolism regulatory effects on murine RAW264.7 macrophages. Int Immunopharmacol 71:198–204. https://doi.org/10.1016/j.intimp.2019.03.023
Shanmugam G, Narasimhan M, Sakthivel R, Kumar RR, Davidson C, Palaniappan S, Claycomb WW, Hoidal JR, Darley-Usmar VM, Rajasekaran NS (2016) A biphasic effect of TNF-α in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox Biol 9:77–89. https://doi.org/10.1016/j.redox.2016.06.004
Ciamporcero E, Daga M, Pizzimenti S, Roetto A, Dianzani C, Compagnone A, Palmieri A, Ullio C, Cangemi L, Pili R, Barrera G (2018) Crosstalk between Nrf2 and YAP contributes to maintaining the antioxidant potential and chemoresistance in bladder cancer. Free Radic Biol Med 115:447–457. https://doi.org/10.1016/j.freeradbiomed.2017.12.005
Acknowledgements
This work was supported by National Natural Science Foundation of China: 81570956; 31600757; 81870744.
Funding
This work was supported by National Natural Science Foundation of China: 81570956; 31600757; 81870744.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, P., Yang, B., Wu, Y. et al. YAP1 regulates chondrogenic differentiation of ATDC5 promoted by temporary TNF-α stimulation through AMPK signaling pathway. Mol Cell Biochem 474, 209–218 (2020). https://doi.org/10.1007/s11010-020-03846-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03846-z