Abstract
The hydrogen electrosorption process was examined in 6 M KOH on Pd binary alloys, containing Rh, Ru, and Pt. Pd-alloys were electrochemically deposited on Au substrate. The electrodes were subjected to activation procedure—hydrogen pretreatment procedure (HPP) at first in 0.5 M H2SO4 and then in 6 M KOH. It was noticed that it was possible to achieve comparable reversibility of hydrogen electrosorption process in acid and in concentrated base. The obtained values of the α→β phase transition potential, hysteresis extent, and maximum hydrogen absorption capacity show good agreement with the data from acidic medium. The observed kinetics of hydrogen electrosorption were strongly hindered in concentrated alkaline media, whereas the influence of the electrolyte on the thermodynamic functions of hydrogen absorption is less pronounced.

Graphical abstract
Similar content being viewed by others
Introduction
In spite of the fact that hydrogen sorption properties of Pd and its alloys are examined for many years, still many aspects of this process are not explained or confusing. Researchers are mostly focused on the synthesis of new electrode material and hydrogen sorption from gas phase [1,2,3,4], wherein the impact of the electrolyte is often overlooked. Therefore, in the literature, there are many publications dedicated to hydrogen electrosorption in Pd-based electrodes from acidic water solutions [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Unfortunately, not many researchers address the problem of hydrogen sorption in alkaline media [22,23,24,25,26,27,28,29,30,31,32,33,34,35] being confined predominantly to Pd examination in diluted solutions.
Moreover, there are limited works concerning the hydrogen sorption in Pd from non-aqueous media [36,37,38,39]. The behavior of Pd-based materials in concentrated alkaline media seems to be very important having regard for the fact that Pd and its alloys are considered as efficient modifiers of hydrogen sorption properties of AB-type alloys [40,41,42]. It was recently found that hydrogen sorption kinetics are strongly enhanced when the surface of an AB5 alloy is modified with Pd nanoparticles [43]. Since hydride batteries still work in concentrated alkaline media, it is crucial to use these electrolytes to examine the process of hydrogen sorption in Pd alloys.
Pd-Rh and Pd-Ru alloys belong to the class of contracted alloys (lattice constant decrease after alloying), whereas Pd-Pt alloys are classified as expanded alloys (lattice constant increase after alloying) [44]. Generally, the assignment to the specific class can be done on the basis of the variation of the α→β phase transition potential with the content of the alloying metal. In case of contracted alloys, the potential of α→β phase transition decrease with the addition of the alloying metal, and in expanded alloys, the behavior is opposite. Pd-Pt alloys are an exception to that rule, since Pd alloying with Pt causes the expansion of the lattice constant, but α→β phase transition potentials are shifted into lower values compared to Pd. Noh et al. assigned this behavior to the decrease in compressibility [45], and Moysan et al. claims that it is the effect of large broadening of the valence band upon alloying [46].
Alloying Pd with Ru or Rh enhances the hydrogen storage capacity since Ru or Rh acts as an electron acceptor to Pd [14, 15, 47,48,49]. Both mentioned types of binary alloys have similar electronic structure, with free states around and below Pd Fermi level. It allows for the electron transfer from Pd to Rh or Ru, resulting in an increase of the number of holes in Pd d-band, that can be filled by the electrons from absorbed hydrogen [50].
Recently, the authors have published the results concerning hydrogen electrosorption in Pd-LVE (limited volume electrode) electrode immersed in concentrated alkaline media [51]. It was noticed that neither the type of concentrated electrolyte (KOH, NaOH, mixed KOH and LiOH, 0.5 M H2SO4) nor long cycling in hydrogen region affected the maximum hydrogen capacity of Pd (H/Pd). It was concluded also that the activation procedure (so-called hydrogen pretreatment procedure (HPP)) has a significant impact on the α→β phase transition potential. After implementation of HPP, α→β phase transition potential in Pd equals to ca. 0.05 V regardless of the type of electrolyte. It was found that subsequent cycling of the Pd-LVE electrode in the potential region corresponding to hydrogen sorption and desorption results in similar values of Gibbs energy for hydrogen absorption. These interesting conclusions were a motivation to subsequent examination of the hydrogen sorption properties in Pd binary alloys with Rh, Ru, and Pt in concentrated alkaline media.
The results obtained for binary alloys in concentrated alkaline media were compared either with those obtained for the same samples in acidic media or with published data. In the view of the authors, studies concerning hydrogen electrosorption properties of Pd binary (Pd alloyed with Rh or Ru or Pt) alloys in concentrated alkaline media have not previously been published.
Experimental
Hydrogen electrosorption experiments were carried out in a three electrode system: Pd-M (M = Rh, Ru, Pt) LVE [51] was the working electrode, Pt gauze was the auxiliary electrode, Hg|HgO|6 M OH− was the reference electrode. LVE electrodes were electrodeposited from the PdCl2 solution with either H2PtCl6, RhCl3, and RuCl3 solutions according to the procedures described in details in [12,13,14,15]. Physicochemical characteristics of the alloys are described in details in [52]. The compositions of each alloy were analyzed with the use of optical emission spectrometry with inductively coupled plasma (ICP-OES) in the analytical laboratory of Mint-Metals LLC (Poland). Before the ICP-OES analysis, the samples were dissolved in aqua regia. The thickness of the LVE electrodes is equal to ca. 0.5 ± 0.1 μm. The hydrogen electrosorption in Pd binary alloys was conducted at first in 0.5 M H2SO4 solution and then in 6 M KOH solution. During the experiments, Ar was used to the deaeration. All potentials were calculated in reference to RHE. The potential recalculation into RHE was done with the use of Hydroflex electrode [38, 39, 51].
Results and discussion
The implementation of the HPP
In previously published research, concerning the behavior of Pd-LVE electrode in concentrated alkaline media, the authors ascertained the impact of HPP on hydrogen sorption properties of the Pd electrodes [51]. The implementation of similar procedures has been checked in case of Pd-binary alloys containing Rh, Ru, and Pt. HPP involves electrode polarization in the potential region of hydrogen absorption and desorption. The techniques of chronoamperometry (CA) and cyclic voltammetry (CV) were used alternately to achieve invariable CA and CV courses. In this study, before HPP in 6 M KOH, the electrodes were subjected to HPP in 0.5 M H2SO4. The results of HPP in acid are shown in inset in Fig. 1a. In case of HPP in acid, only a few cycles/steps lead to stabilization of hydrogen oxidation potential (EHox).
The greatest discrepancy was visible for Pd electrode; however, after ca. 6 cycles, the EHox reaches stable value of ca. 0.10 V. EHox value lower than in Pd was achieved for different binary alloys, irrespective of the type of the alloying metal and composition. Figure 1a presents further electrodes cycling in 6 M KOH. One can notice that in case of some electrodes, EHox is lower at the beginning of the experiment than after a few cycles/steps of HPP. This behavior was observed for all of the investigated alloys, regardless of the alloying metal. Similarly as for Pd electrode, in alloys, CA has a stronger impact on the evaluation of the EHox towards lower potentials than CV. Analysis of the HPP course in Fig. 1a leads to the conclusion that rather deterioration than improvement of reversibility of hydrogen sorption process can be observed after application of CV. It is manifested by the increase of EHox in subsequent CV cycles. Evaluation of the dependence of EHox on number of CV cycles shows that for the alloys lower final value of EHox (ca. 0.10–0.12 V) can be achieved than for Pd. Figure 1b presents the CV behavior for exemplary Pd-Ru electrode before and after HPP in 6 M KOH.
It is clearly visible that application of HPP results in a significant increase of the electrochemical reversibility of the hydrogen sorption process. CV after HPP in 6 M KOH resembles the course of the CV in acid.
HEI for Pd binary alloys
After implementation of HPP in concentrated alkaline media, Pd binary alloys were subjected to the experiments of hydrogen absorption and desorption with the use of CA (absorption, desorption) and CV (absorption in wide potential range) techniques. In Fig. 2a–c, there are results obtained in acid and concentrated base for the selected compositions of Pd-Rh, Pd-Ru, and Pd-Pt alloys. It should be emphasized that electrolytes used in hydrogen electrosorption experiments significantly vary in pH and viscosity. Dynamic viscosity of 6 M KOH is 2 times higher than the one of 0.5 M H2SO4. Furthermore, in both cases, the source of hydrogen is different: hydrogen cation in acid, water molecule in base. Despite the differences in electrolytes used in the experiments, the courses of hydrogen electrosorption isotherms (HEIs) in the two-phase (α↔β) region show good compatibility. Similar conclusions were drawn previously for Pd-LVE [51]. It is worth noting that achievement of these results was possible only after implementation of HPP.
α→β phase transition potential and hydrogen electrosorption hysteresis
From HEIs it was possible to designate the values of α→β and β→α phase transition potentials in selected Pd binary alloys. Figure 3a shows the dependence of α→β phase transition potentials on the Pd content in the bulk. For comparison, there are also presented, the results obtained for the same electrodes in acid. Eα→β vs Pd content dependence is linear in acid as well as in base. It means that the use of Eα→β to estimate the approximate composition of binary alloys can be utilize successfully also in concentrated alkaline media. Unfortunately, the relation of Eβ→α to Pd content shows worse agreement; however, it is also visible in acidic media. The discrepancies of Eβ→α vs Pd content can be explained based on the conditions of CA desorption measurements. To obtain full saturation of hydrogen, the electrode was polarized for several dozen/several hundred seconds in the potential of β-phase formation. These conditions of electrode polarization results in not only hydrogen saturation but also undesirable impurity adsorption on the electrode surface. Since in concentrated alkaline solution, in principle, the concentration of impurities is higher than in acid, this effect is noticeable more often in basic than in acidic media. The strongest dependence of Eα→β on Pd content is noticeable in case of Pd-Ru alloys, while for Pd-Rh and Pd-Pt, these relations have similar courses. From Fig. 3b, one can see that the hysteresis extent of hydrogen sorption decreases the fastest with increasing Pd content in Pd-Ru alloy. Pd-Pt alloys reveal the weakest dependence of hysteresis on the alloy composition. For Pd-Rh alloys, slope of this dependence is intermediate between Pd-Ru and Pd-Pt alloys. In general, it can be concluded that no significant difference in the phase transition potential of hydrogen sorption, and by association the degree of hysteresis, was noticed after comparison of the results achieved in acid and concentrated base for Pd binary alloys. After implementation of HPP, the behavior of Pd-binary alloys in two-phase region in concentrated alkaline media is similar to the one obtained in acid.
The kinetics of hydrogen absorption in concentrated alkaline media
The kinetics of hydrogen absorption in concentrated alkaline media were studied through the analysis of hydrogen absorption times determined from CA absorption curves. Figure 4a presents the influence of hydrogen sorption potential on the time of absorption and desorption in base and acid for the exemplary Pd-Ru alloy. One can notice that in the absorption and desorption processes, times of hydrogen sorption are significantly longer in base than in acid. This effect is more pronounced in regions where the α and β phases coexist. Thus, further discussion on the hydrogen sorption kinetics was based on the variation of the maximum times of hydrogen sorption with the alloys composition. Regarding the fact that absorption times can be influenced by the amount of absorbed hydrogen, times of absorption were normalized to the H/M, according to the procedure described in [14]. It can be noticed from Fig. 4b that normalized times of absorption in concentrated base are generally few times higher than in acid. They are the longest in case of Pd-Ru alloys (max. 9 times higher than in acid) and Pd-Pt alloys (max. 3 times higher than in acid) electrodes. However, overall tendency to reduce the time of absorption with increasing content of the alloying metal is preserved, as it is in case of acid. The increase of the sorption time in concentrated alkaline media can be an effect of the presence of impurities on the electrode surface (mentioned in the “α→β phase transition potential and hydrogen electrosorption hysteresis” section). Since the individuals adsorbed on the electrode surface can hinder the process of hydrogen insertion (removal) to (from) bulk of the electrode. Other arguments confirming deterioration of the kinetics of hydrogen sorption in concentrated base are related to the reduced value of the maximum hydrogen desorption currents (compared to results in acid). To verify proposed hypothesis, simple experiment was conducted—before the procedure of CA hydrogen desorption, the electrode was polarized in the potential region involving creation of surface oxides and the reduction of them. Before and after this experiment, CA desorption curve was registered (after full saturation of electrode). It was noticed that the desorption time and the max. desorption current were higher after the electrode polarization in “surface oxide region.” It indicates that the hydrogen sorption kinetics are strongly influenced by the state of the electrode surface. However, the applied procedure cannot be utilized often, since many cycles in surface oxide region causes the alloy dissolution and thereby the change in the alloy composition [53].
a The dependence of time of hydrogen sorption on potential value for 98% Pd-Ru; hydrogen desorption, open symbols; hydrogen absorption, filled symbols. b The influence of alloy composition and type of electrolyte on the maximum, normalized time of absorption; hydrogen sorption in acid, open symbols; hydrogen sorption in base, filled symbols
The maximum hydrogen capacity of the alloy in the concentrated alkaline media
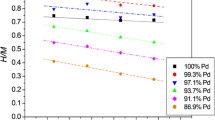
From the point of view concerning the hydrogen storage properties, it is very important to discuss the influence of the type of the electrolyte and the alloying metal on the maximum hydrogen capacity. Figure 5 presents the results obtained for Pd binary alloys with Rh, Ru, and Pt in acid and concentrated base. For comparison, there were placed also the literature data registered in acid [12,13,14]. One can notice that the values of maximum hydrogen capacity obtained in acid and concentrated base are similar. Thus, the tendency of decreasing H/Mmax with increasing content of the Pd-alloying metal is also noticeable. It should be mentioned that for Pd-Rh alloy, containing ca. 92.5% Pd, the H/M in concentrated base is also (as in acid) higher than in pure Pd. For Pd-Ru alloys, this property has not been confirmed, since, in acid, it was characteristic only for alloys containing less than 1% of Ru [14]. These results show that surface processes (individuals adsorbed on the electrode surface) have no adverse influence on the maximum hydrogen capacity. In case of all studied Pd-Pt alloys, slight increase of the H/Mmax can be observed in 6 M KOH compared to acid. However, it can be noticed (comparing with the literature data) for examined samples, not only in concentrated base, but also in acid. This behavior can be explained on the basis of the theory postulated in the literature [54] that hydrogen capacity increases with the decreasing thickness of the LVE. The thicknesses of examined samples were ca. 0.5 μm, while thicknesses of the electrodes from the literature were ca. 1 μm. In case of Pd-Rh and Pd-Ru alloys, there is rather slight decrease of hydrogen maximum capacity in electrodes examined in this study. In Table 1, there are calculated the values of maximum hydrogen solubility of hydrogen in α-phase and minimum hydrogen solubility in β-phase for Pd binary alloys enable to β-phase formation. These values give ability to conclude about the extent of the miscibility gap in Pd-Rh-H, Pd-Ru-H, and Pd-Pt-H systems. Comparing them to the results from the literature [12,13,14], hydrogen solubilities in αmax and βmin in each of the system studied are lower than in acid. Nevertheless, it does not influence on the average extent of the two-phase region. Moreover, the closure of the miscibility gap in concentrated base for Pd-Rh and Pd-Ru alloys follows for similar compositions as in acid. It is ca. 74% Pd in Pd-Rh alloys (ca. 75% in acid [13]) and 88% Pd for Pd-Ru alloys (ca. 93% in acid [14]). The intersection point of the curves delimiting the two-phase area in Pd-Pt-H system indicates that the closure of the two-phase area follows for ca. 56% of Pd content, whereas it is ca. 85% Pd in acid [12]. The discrepancies result mostly from the less, than in acid, impact of the alloying metal addition on the values of hydrogen concentration in βmin phase. In case of acid, the H/Mβmin values vary stronger with the change in the alloy composition; thus, the closure of the miscibility gap follows in acid for the higher contents of Pd than in concentrated base. Moreover, for Pd-Pt-H system, the calculations were done for alloys containing less Pd than in Pd-Rh and Pd-Ru alloys. The inflexion points in CA desorption and absorption curves used for calculations of hydrogen concentration in both phases are better outlined for alloys containing more Pd. Additionally, in concentrated alkaline media the shapes of CA curves are “less sharp,” what could be also a reason for the discrepancy.
The thermodynamics of hydrogen sorption in alloys in the concentrated alkaline media
The values of Eα→β and their evaluation with the temperature can be used to calculate the values of thermodynamic functions: Gibbs energy (Table 2 for 298 K), enthalpy, and entropy (Fig. 6) for hydrogen sorption process in concentrated alkaline media. For pure Pd in concentrated base, values of thermodynamic functions for hydrogen absorption are as follows: ΔGα→β = − 9.6 kJ mol−1H2, ΔHα→β = – 38.3 kJ mol−1H2, ΔSα→β = – 96.5 J mol−1H2 K−1. ΔGα→β values obtained for binary alloys show that the least stable hydrides are formed in case of Pd-Ru alloys, whereas the stability of hydrides in Pd-Rh and Pd-Pt is similar. Figure 6 presents the relative values of ΔHα→β and ΔSα→β for examined Pd binary alloys. In general, the process of hydrogen sorption is less exothermic in alloys than in pure Pd. ΔHα→β values increase slightly with the addition of the alloying metal, which indicates that the hydrogen sorption process becomes less exothermic in alloys containing more Ru, Rh, and Pt. In case of the thermodynamic data obtained for binary alloys in acid, the dependence of ΔHα→β on the addition of alloying metal are stronger outlined that in concentrated base, especially for Pd-Ru alloys [12, 15, 18]. The variation of ΔSα→β values with the content of alloying metal is used in literature to determine the type of interstices occupied by hydrogen. The lack of dependence signifies that hydrogen can occupy both available types of interstices i.e. near Pd and near alloying metal, whereas the variation of ΔSα→β with the addition of alloying metal indicates that hydrogen occupy preferably the interstices near the Pd atoms. The dependence obtained in concentrated base for binary alloys rather indicates that hydrogen locates in both, available type of interstices (near Pd atoms and near alloying metal atoms) although still prefers Pd atoms neighborhood. These results are in line with the literature data for Pd-Pt [12] and Pd-Rh [18] alloys. However, in case of Pd-Ru alloys in acid strong increase of ΔSα→β with the addition of alloying metal was observed [15]. Unfortunately, there is a lack of thermodynamic data in literature for that system, especially for hydrogen sorption from gas phase.
Conclusions
Application of electrode activation procedure (HPP) in Pd binary alloys with Rh, Ru, and Pt in 6 M KOH leads to the achievement of reversibility of hydrogen electrosorption process, comparable with this in 0.5 M H2SO4. Pd alloys require fewer cycles/steps of HPP than pure Pd. After application of HPP, essential hydrogen electrosorption properties such as phase transition potential, hysteresis, and hydrogen maximum capacity are similar to those obtained in acid. In concentrated alkaline media the kinetics of hydrogen electrosorption are hindered, what is mostly caused by the presence of the surface impurities. The use of concentrated alkaline electrolyte influences slightly (in comparison with acid) the thermodynamics of hydrogen electrosorption process. The obtained results indicate that Pd-binary alloys with Rh, Ru, and Pt can be effectively used to modification of AB type alloys, commonly working in concentrated alkaline media.
References
Flanagan TB, Luo S (2007) Thermodynamics of hydrogen solution and hydride formation in binary Pd alloys. Phs Eqil Diff 28(1):49–57
Yang N, Yee JK, Zhang Z, Kurmanaeva L, Cappillino P, Stavila V, Lavernia EJ, San Marchi C (2015) Hydrogen sorption characteristics of nanostructured Pd–10Rh processed by cryomilling. Acta Mater 82:41–50
Martínez de Yuso A, Oumellal Y, Zlotea C, Vidal L, Ghimbeua CM (2017) In-situ Pd–Pt nanoalloys growth in confined carbon spaces and their interactions with hydrogen. Nano-Struct Nano-Objects 9:1–12
Liu J, Bellini S, Niek CA, de Nooijer NCA, Li H, Gallucci F, Caravella A (2020) Hydrogen permeation and stability in ultra-thin Pd-Ru supported membranes. Int J Hydrog Energy 5:7455–7467
Millet P, Srour M, Faure R, Durand R (2001) A study of hydrogen absorption and desorption reactions in palladium electrodes using the potential step method. Electrochem Commun 3(9):478–482
Gabrielli C, Grand PP, Lasia A, Perrot H (2002) Study of the hydrogen/palladium system by fast quartz microbalance techniques. Electrochim Acta 47(13-14):2199–2207
Comisso N, De Ninno A, Del Giudice E, Mengoli G, Soldan P (2004) Electrolytic hydriding of Pd79.5Rh20.5 alloy. Electrochim Acta 49(9-10):1379–1388
Gabrielli C, Grand PP, Lasia A, Perrot H (2004) Investigation of hydrogen adsorption and absorption in palladium thin films. II. Cyclic voltammetry. J Electrochem Soc 151(11):A1925–A1936
Vigier F, Jurczakowski R, Lasia A (2006) Determination of hydrogen absorption isotherm and diffusion coefficient in Pd81Pt19 alloy. J Electroanal Chem 588(1):32–43
Lasia A, Jurczakowski R, Łosiewicz B (2007) Kinetic and Thermodynamic Parameters of Hydrogen Sorption in Pd, Pd-Pt and on Pt. ECS Trans 2:11–19
Duncan H, Lasia A (2008) Separation of hydrogen adsorption and absorption on Pd thin films. Electrochim Acta 53(23):6845–6850
Hubkowska K, Łukaszewski M, Czerwiński A (2011) Influence of temperature on hydrogen electrosorption into palladium-noble metal alloys. Part 2 - Palladium-platinum alloys. Electrochim Acta 56(5):2344–2350
Koss U, Łukaszewski M, Hubkowska K, Czerwiński A (2011) Influence of rhodium additive on hydrogen electrosorption in palladium-rich Pd-Rh alloys. J Solid State Electrochem 15(11-12):2477–2487
Hubkowska K, Koss U, Łukaszewski M, Czerwiński A (2013) Hydrogen electrosorption into Pd-rich Pd-Ru alloys. J Electroanal Chem 704:10–18
Hubkowska K, Łukaszewski M, Czerwiński A (2014) Thermodynamics of hydride formation and decomposition in electrodeposited Pd-rich Pd-Ru alloys. Electrochem Commun 48:40–43
Sheridan LB, Yates VM, Benson DM, Stickney JL, Robinson DB (2014) Hydrogen sorption properties of bare and Rh-modified Pd nanofilms grown via surface limited redox replacement reactions. Electrochim Acta 128:400–405
Uluc AV, Mol JMC, Terryn H, Böttger AJ (2014) Hydrogen sorption and desorption related properties of Pd-alloys determined by cyclic voltammetry. J Electroanal Chem 73415:53–60
Koss U, Łukaszewski M, Hubkowska K, Czerwiński A (2015) Thermodynamic aspects of hydrogen electrosorption into Pd-Rh alloys. J Electroanal Chem 756:124–130
Zalineeva A, Baranton S, Coutanceau C, Jerkiewicz G (2017) Octahedral palladium nanoparticles as excellent hosts for electrochemically adsorbed and absorbed hydrogen. Sci Adv 3(2):e1600542
Łosiewicz B, Lasia A (2018) Study of the hydrogen absorption/diffusion in Pd80Rh20 alloy in acidic solution. J Electroanal Chem 822:153–162
Konda SK, Mona Amiri M, Chen A (2017) Significant enhancement of electrosorption of hydrogen into palladium via a facile annealing process. Int J Hydrog Energy 42(17):12375–12383
Rubeš I, Bilyková H (1972) Activity of palladium electrode in alkaline medium Collection Czech Chem Commun 37(3):715–724
Burke LD, Casey JK (1993) An Examination of the Electrochemical Behavior of Palladium Electrodes in Acid. J Electrochem Soc 140(5):1292–1298
Hu CC, Wen TC (1994) Voltammetric Investigation of Hydrogen Sorption/Desorption at/within Oxide‐Derived Pd Electrodes in NaOH and H2SO4. J Electrochem Soc 141(11):2996–3001
Hu CC, Wen TC (1995) Effects of pH and Anion on Hydrogen Sorption/Desorption at/within Oxide‐Derived Pd Electrodes. J Electrochem Soc 142(5):1376–1383
Czerwiński A, Maruszczak G, Żelazowska M, Łańcucka M, Marassi R, Zamponi S (1995) The absorption of hydrogen and deuterium in thin palladium electrodes Part III: The influence of solution composition. J Electroanal Chem 386(1-2):207–211
Czauderna M, Maruszczak G, Czerwiński A (1995) Use of neutron activation analysis for the determination of cesium in Pd electrodes on Pt and Au matrices. J Radioanal Nucl Chem 199(5):375–383
Yang TH, Pyun SI (1996) Hydrogen absorption and diffusion into and in palladium: ac-impedance analysis under impermeable boundary conditions. Electrochem Acta 41(6):843–848
Paillier J, Roué L (2005) Nanostructured Palladium Thin Films Prepared by Pulsed Laser Deposition: Structural Characterizations and Hydrogen Electrosorption Properties. J Electrochem Soc 152(1):E1–E8
Martin MH, Lasia A (2008) Study of the hydrogen absorption in Pd in alkaline solution. Electrochim Acta 53:6317–6322
Martin MH, Lasia A (2009) Hydrogen sorption in Pd monolayers in alkaline solution. Electrochim Acta 54:5292–5299
Nagle LC, Burke LD (2010) Anomalous electrochemical behaviour of palladium in base. J Solid State Electrochem 14:1465
Shin JW, Bertocci U, Stafford GR (2011) In Situ Stress Measurement During Hydrogen Sorption on Ultrathin (111)-Textured Pd Films in Alkaline Electrolyte. J Electrochem Soc 158:F127–F134
Montero MA, Gennero de Chialvo MR, Chialvo AC (2018) Unexpected Behavior of the Hydrogen Oxidation Reaction on Palladium in Alkaline Solution: A Feasible Kinetic Explanation J Electrochem Soc 165:J3069–J3073
Oliveira VL, Eric Sibert E, Soldo-Olivier Y, Ticianelli EA, Chatenet M (2018) Insertion/Disinsertion of Hydrogen in Tailored Pd Layers Deposited on Pt(111) Surface in Alkaline and Acidic Medium. Electrocat 9(2):258–263
Tremblay J, Nguyen NL, Rochefort D (2013) Hydrogen absorption by a palladium electrode from a protic ionic liquid at temperatures exceeding 100 °C. Electrochem Commun 34:102–104
Meng T, Young KH, Wong DF, Nei J (2017)Ionic Liquid-Based Non-Aqueous Electrolytes for Nickel/Metal Hydride Batteries Batteries 3(4):4
Pająk M, Hubkowska K, Czerwiński A (2018) The study of hydrogen sorption in palladium limited volume electrode from DEMA-TFO ionic liquid. J Electroanal Chem 825:73–76
Pająk M, Hubkowska K, Czerwiński A (2019) Nitrate protic ionic liquids as electrolytes: Towards hydrogen sorption in Pd. Electrochim Acta 324:134851
Barsellini D, Visintin A, Triaca WE, Soriaga MP (2003) Electrochemical characterization of a hydride-forming metal alloy surface-modified with palladium. J Power Sources 124(1):309–313
Ambrosio RC, Ticianelli EA (2005) Studies on the influence of palladium coatings on the electrochemical and structural properties of a metal hydride alloy. Surf Coat Technol 197(2-3):215–222
Visintin A, Castro EB, Real SG, Triaca WE, Wang C, Soriaga MP (2006) Electrochemical activation and electrocatalytic enhancement of a hydride-forming metal alloy modified with palladium, platinum and nickel. Electrochim Acta 51:3658–3667
Hubkowska K, Soszko M, Krajewski M, Czerwiński A (2019) Enhanced kinetics of hydrogen electrosorption in AB5 hydrogen storage alloy decorated with Pd nanoparticles. Electrochem Commun 100:100–103
Thiébaut S, Bigot A, Achard JC, Limacher B, Leroy D, Percheron-Guegan A (1995) Structural and thermodynamic properties of the deuterium-palladium solid solutions systems: D2-[Pd(Pt), Pd(Rh), Pd(Pt, Rh)]. J Alloys Compd 231(1-2):440–447
Noh H, Flanagan TB, Sonoda T, Sakamoto Y (1995) Solubility and thermodynamics of hydrogen in homogeneous f.c.c. Pd-Pt alloys. J Alloys Compd 228(2):164–171
Moysan I, Paul-Boncour V, Thiebaut S, Sciora E, Fournier JM, Cortes R, Bourgeois S, Percheron-Guegan A (2001) Pd–Pt alloys: correlation between electronic structure and hydrogenation properties. J Alloys Compd 332:14–20
Fazle Kibria AKM, Sakamoto Y (2000) The effect of alloying of palladium with silver and rhodium on the hydrogen solubility, miscibility gap and hysteresis. Int J Hydrog Energy 25(9):853–859
Frolich K, Severin HG, Hemplemann R, Wicke E (1980) Local Magnetic Moments of Ruthenium in Palladium/Ruthenium/Hydrogen Alloys. Z Phys Chem N F 119(1):33–52
Fujii K, Ishimatsu N, Maruyama H, Shishidou T, Hayakawa S, Kawamura N (2017) Relationship between element-selective electronic states and hydrogen absorption properties of Pd-M (M = Ru, Rh, Ag, and Au) alloys. Phys Rev B95:024116
Wicke E, Frolich K (1989) Electronic and Elastic Effects in the Phase Diagrams of Binary Pd Alloy Hydrides. Z Phys Chem N F 163(Part_1):35–40
Hubkowska K, Soszko M, Symonowicz M, Łukaszewski M, Czerwiński A (2017) Electrochemical Behavior of a Pd Thin Film Electrode in Concentrated Alkaline Media. Electrocat 8(4):295–300
Hubkowska K, Łukaszewski M, Soszko M, Koss U, Hamankiewicz B, Czerwiński A (2018) Comparative physicochemical and electrochemical characterization of the structure and composition of thin Pd binary and ternary codeposits with Pt, Ru, and Rh. Materials 11(5):798–821
Hubkowska K, Themsirimongkon S, Saipanya S, Łukaszewski M, Czerwiński A (2020) The Modification of Electrochemical Properties of Pd by its Alloying with Ru, Rh, and Pt: the Study of Ternary Systems. Electrocat 11(2):247–257
Czerwiński A, Kiersztyn I, Grdeń M, Czapla J (1999) Study of hydrogen sorption in palladium limited volume electrodes (Pd-LVE). I. Acidic solutions. J Electroanal Chem 471(2):190–195
Funding
This work was financially supported by the National Science Centre (NCN, Poland) grant no. 2015/17/B/ST8/03377 (ID 289956).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors dedicate this article to Professor Fritz Scholz on the occasion of his 65th birthday in recognition of his achievements and wishing him further scientific and publishing success.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hubkowska, K., Czerwiński, A. Tuning hydrogen sorption properties of Pd by its alloying with Ru, Rh, and Pt: the study of binary alloys in concentrated alkaline media. J Solid State Electrochem 24, 3135–3143 (2020). https://doi.org/10.1007/s10008-020-04776-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04776-y