Abstract
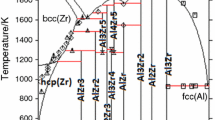
Using CALPHAD method, the Al-Zr, Al-Li and Al-Li-Zr systems have been reassessed based on the latest experimental phase relations from literatures and the first-principle calculation of the formation enthalpy for ternary compound T1(AlLi2Zr) in this work. The excess Gibbs energies of solution phases, including liquid, bcc, fcc and hcp, were expressed by the Redlich–Kister polynomial. The stoichiometric compounds, i.e. Al4Zr5, Al3Zr5, AlZr2, AlZr3, Al2Li3, Al4Li9 and T1(AlLi2Zr), were modeled as stoichiometric model and the non-stoichiometric compounds, i.e. Al3Zr, Al2Zr, Al3Zr2, AlZr, Al3Zr4, Al2Zr3, AlLi2 and T2(AlLix+yZr5−x), were described with different sublattice models. Finally, a set of reasonable thermodynamic parameters for Al-Li-Zr ternary system have been obtained, which notes a clear improvement on the self-consistency. The calculated isothermal section at 470 K of Al-Li-Zr system was in reasonable agreement with the experimental one.






Similar content being viewed by others
Data Availability
The raw data and the processed data required to reproduce these findings are available from the corresponding author upon request, but most of them are included within the article.
References
T. Tian, X.F. Wang, and W. Li, Ab Initio Calculations on Elastic Properties in L12 Structure Al3X and X3Al-type (X = Transition or Main Group Metal) Intermetallic Compounds, Solid State Commun., 2013, 156, p 69-75. https://doi.org/10.1016/j.ssc.2012.10.021
Y. Wang, H.Y. Liu, X.C. Ma, R.Z. Wu, J.F. Sun, L.G. Hou, J.H. Zhang, X.L. Li, and M.L. Zhang, Effects of Sc and Zr on Microstructure and Properties of 1420 Aluminum Alloy, Mater. Charact., 2019, 154, p 241-247. https://doi.org/10.1016/j.matchar.2019.06.001
Y.J. Gao, Q.F. Mo, H.N. Chen, C.G. Huang, and L.N. Zhang, Atomic Bonding and Mechanical Properties of Al-Li-Zr Alloy, Mater. Sci. Eng. A, 2009, 499, p 299-303. https://doi.org/10.1016/j.msea.2007.11.113
M.H. Tosten, J.M. Galbraith, and P.R. Howell, Nucleation of δ′(AI3Li) on/β′(Al3Zr) in Al-Li-Zr and AI-Li-Cu-Zr Alloys, J. Mater. Sci. Lett., 1987, 6, p 51-53. https://doi.org/10.1007/BF01729426
Y. Wang, H.J. Wu, X.T. Liu, Y.L. Jiao, J.F. Sun, R.Z. Wu, L.G. Hou, J.H. Zhang, X.L. Li, and M.L. Zhang, High-Strength and Ductility Bimodal-Grained Al-Li/Al-Li-Zr Composite Produced by Accumulative Roll Bonding, Mater. Sci. Eng. A, 2019, 761, p 138049. https://doi.org/10.1016/j.msea.2019.138049
J.O. Andersson, T. Helander, L. Hoglund, P. Shi, and B. Sundman, Thermo-Calc and DICTRA, Computational Tools for Materials Science, CALPHAD, 2002, 26, p 273-312. https://doi.org/10.1016/S0364-5916(02)00037-8
L. Zhang, M. Stratmann, Y. Du, B. Sundman, and I. Steinbach, Incorporating the CALPHAD Sublattice Approach of Ordering into the Phase-Field Model with Finite Interface Dissipation, Acta Mater., 2015, 88, p 156-169. https://doi.org/10.1016/j.actamat.2014.11.037
B. Samanta, S. Balakrishnan, R. Pagoti, K. Ananthasivan, K. Joseph, and A. Dasgupta, Measurement of Solidus and Liquidus in the System Zr-Al (20–95 at.% Zr) by Using the Spot-Technique, Thermochim. Acta, 2018, 667, p 132-139. https://doi.org/10.1016/j.tca.2018.07.009
K. Puhakainen, M. Boström, T.L. Groy, and U. Häussermann, A New Phase in the System Lithium–Aluminum: Characterization of Orthorhombic Li2Al, J. Solid State Chem., 2010, 183, p 2528-2533. https://doi.org/10.1016/j.jssc.2010.08.029
G.M. Zatorska, V.V. Pavlyuk, and V.M. Davydov, Phase Equilibria and Crystal Structure of Compounds in the Zr-Li-Al System at 470 K, J. Alloys Compd., 2002, 333, p 138-142. https://doi.org/10.1016/S0925-8388(01)01700-5
N. Saunders and V.G. Rivlin, Thermodynamic Characterization of Al-Cr, AI-Zr and Al-Cr-Zr Alloy Systems, Mater. Sci. Technol., 1986, 2, p 521-527. https://doi.org/10.1179/mst.1986.2.6.520
N. Saunders, Calculated Stable and Metastable Phase Equilibria in Al-Li-Zr Alloys, Z. Metallkd., 1989, 80, p 894-903
J. Murray, A. Peruzzi, and J.P. Abriata, The AI–Zr (Aluminum–Zirconium) System, J. Phase Equilib., 1992, 13, p 277-291. https://doi.org/10.1007/BF02667556
T. Wang, Z.P. Jin, and J.C. Zhao, Thermodynamic Assessment of the Al-Zr Binary System, J. Phase Equilib., 2001, 22, p 544-551. https://doi.org/10.1007/s11669-001-0072-4
E. Fischer and C. Colinet, An Updated Thermodynamic Modeling of the Al-Zr System, J. Phase Equilib., 2015, 36, p 404-413. https://doi.org/10.1007/s11669-015-0398-y
M. Tamim and K. Mahdouk, Thermodynamic Reassessment of the Al-Zr Binary System, J. Therm. Anal. Calorim., 2018, 131, p 1187-1200. https://doi.org/10.1007/s10973-017-6635-3
B. Hallstedt and O. Kim, Thermodynamic Assessment of the Al-Li system, Z. Metall., 2007, 98, p 961-969. https://doi.org/10.3139/146.101553
Z.H. Long, S.Y. Liang, and F.C. Yin, Experimental Study and Thermodynamic Assessment of Li-Si-Zr Ternary System, Chin. J. Nonferrous Met., 2015, 25, p 1227-1235
D.J. McPherson and M. Hansen, The System Zirconium Aluminum, Trans. Am. Soc. Met., 1954, 46, p 354-374
M. Pötzschke and K. Schubert, The Constitution of Some T4–B3 Homologuos and Quasi-Homologuos Systems: II: The Systems Titanium–Aluminium, Zirconium–Aluminium, Hafnium–Aluminium, Molybdenum–Aluminium and Some Ternary Systems, Z. Metallkd., 1962, 53, p 548-561
A. Peruzzi, Reinvestigation of the Zr-Rich End of the Zr-Al Equilibrium Phase Diagram, J. Nucl. Mater., 1992, 186, p 89-99. https://doi.org/10.1016/0022-3115(92)90326-G
W.L. Fink and L.A. Willey, Equilibrium Relations in Aluminum-Zirconium Alloys of High Purity, Trans. AIME, 1939, 133, p 69-80
A. Janghorban, A. Antoni-Zdziobek, M. Lomello-Tafin, C. Antion, Th. Mazingue, and A. Pisch, Phase Equilibria in the Aluminium-Rich Side of the Al-Zr System, J. Therm. Anal. Calorim., 2013, 114, p 1015-1020. https://doi.org/10.1007/s10973-013-3113-4
F. Wang, D.G. Eskin, and A.V. Khvan, On the Occurrence of a Eutectic-Type Structure in Solidification of Al-Zr Alloys, Scr. Mater., 2017, 133, p 75-78. https://doi.org/10.1016/j.scriptamat.2017.02.027
A.V. Khvan, D.G. Eskinb, and K.F. Starodub, New Insights into Solidification and Phase Equilibria in the Al-Al3Zr System: Theoretical and Experimental Investigations, J. Alloys Compd., 2018, 743, p 626-638. https://doi.org/10.1016/j.jallcom.2018.02.023
P. Chiotti and P.F. Woerner, Metal Hydride Reactions. I. Reaction of Hydrogen With Solutes in Liquid Metal Solvents, J. Less-Common Met., 1964, 7, p 111-119. https://doi.org/10.1016/0022-5088(64)90052-9
O. Dezellus, B. Gardiola, and J. Andrieux, On the Solubility of Group IV Elements (Ti, Zr, Hf) in Liquid Aluminum Below 800°C, J. Phase Equilib. Diff., 2014, 35, p 120-126. https://doi.org/10.1007/s11669-013-0278-2
S.N. Tiwari and K. Tangri, The Solid Solubility of Aluminum in α-Zirconium, J. Nucl. Mater., 1970, 34, p 92-96. https://doi.org/10.1016/0022-3115(70)90011-5
T.B. Massalski, H. Okamoto, P.R. Subramanian, and L. Kacprzak, Binary Alloy Phase Diagrams, 2nd ed., ASM International, Materials Park, 1990
C.G. Wilson and E.J. Spooner, The Crystal Structure of ZrAl2, Acta Crystallogr., 1960, 13, p 358-359
C.G. Wilson, D.K. Thomas, and F.J. Spooner, The Crystal Structure of Zr4Al3, Acta Crystallogr., 1960, 13, p 56-57
F.J. Spooner and C.G. Wilson, The Crystal Structure of ZrAl, Acta Crystallogr., 1962, 15, p 621-622
G. Brauer, Crystal Structure of Intermetallic Alloys of Aluminium with Titanium, Zirconium, Thorium, Niobium and Tantalum, Naturwissenschaflen, 1938, 26, p 144
R.J. Kematick and H.F. Franzen, Thermodynamic Study of the Zirconium–Aluminum System, J. Solid State Chem., 1984, 54, p 226-234. https://doi.org/10.1016/0022-4596(84)90150-6
R. Klein, I. Jacob, P.A.G. O’Hare, and R.N. Goldberg, Solution-Calorimetric Determination of the Standard Molar Enthalpies of Formation of the Pseudobinary Compounds Zr(AlxFe1−x)2 at the Temperature 298.15 K, J. Chem. Thermodyn., 1994, 26, p 599-608. https://doi.org/10.1006/jcht.1994.1069
S.V. Meschel and O.J. Kleppa, Standard Enthalpies of Formation of 4d Aluminides by Direct Synthesis Calorimetry, J. Alloys Compd., 1993, 191, p 111-116. https://doi.org/10.1016/0925-8388(93)90280-Z
T. Maciąg, Enthalpy of Formation of Intermetallic Phases from Al–Zr System Determined by Calorimetric Solution Method, J. Therm. Anal., 2018, 134, p 423-431. https://doi.org/10.1007/s10973-017-6917-9
Y.H. Duan, B. Huang, Y. Sun, M.J. Peng, and S.G. Zhou, Stability, Elastic Properties and Electronic Structures of the Stable Zr-Al Intermetallic Compounds: A Frst-Principles Investigation, J. Alloys Compd., 2014, 590, p 50-60. https://doi.org/10.1016/j.jallcom.2013.12.079
J. Wang, S.L. Shang, Y. Wang, Z.G. Mei, Y.E. Liang, Y. Du, and Z.K. Liu, First Principles Calculations of Binary Al Compounds: Enthalpies of Formation and Elastic Properties, J. CALPHAD, 2011, 35, p 562-573. https://doi.org/10.1016/j.calphad.2011.09.009
G. Ghosh and M. Asta, First-Principles Calculation of Structural Energetic of Al-TM (TM = Ti, Zr, Hf) Intermetallics, J. Acta Mater., 2005, 53, p 3225-3252. https://doi.org/10.1016/j.actamat.2005.03.028
H. Zhang and S.Q. Wang, The Structural Stabilities of the Intermetallics and the Solid-State Phase Transformations Induced by Lattice Vibration Effects in the Al-Zr System by First-Principles Calculations, J. Mater. Res., 2010, 25, p 1689-1694. https://doi.org/10.1557/JMR.2010.0227
Y.O. Esin, N.P. Bobrov, M.S. Petrushevski, and P.V. Gel’d, Enthalpies of Formation of Liquid Aluminium Alloys with Titanium and Zirconium, Akad. Nauk. SSSR Met., 1974, 5, p 104-109
V.S. Sudavtsova, G.I. Batalin, and V.S. Tutevich, Thermodynamic Properties of Molten Binary Alloys in Systems Al-Zr (Nb, Mo), Izv. Akad. Nauk. SSSR Met., 1985, 5, p 185-187
V. Witusiewicz, U.K. Stolz, I. Arpshofen, and F. Sommer, Thermodynamics of Liquid Al-Cu-Zr Alloys, Z. Metallkd., 1998, 89, p 704-713
V.S. Sudavtsova and N.V. Podoprigora, Thermodynamic Properties of Melts in Al-TI (Zr, Hf) Binary Systems, Powder Metall. Met. Ceram., 2009, 48, p 83. https://doi.org/10.1007/s11106-009-9091-1
K. Gajavalli, M. Barrachin, and P. Benigni, Determination of Solution Enthalpy of Zirconium in Liquid Aluminum, J. Chem. Thermodyn., 2019, 135, p 198-204. https://doi.org/10.1016/j.jct.2019.03.037
A. Müller, The Al-Li Phase Diagram, Z. Metallkd., 1926, 18, p 231
A. Grube, L. Mohr, and W. Breuning, Elektrische Leitfähigkeit und Zustandsdiagramm bei binären Legierungen. 17. Mitteilung. Das System Lithium–Aluminium, Z. Elektrochem., 1935, 41, p 880-883. https://doi.org/10.1002/bbpc.19350411213
F.I. Shamray and P.Ya. Saldau, Equilibrium Diagram of the System Al-Li, Izv. Akad. Nauk SSSR, Otd. Khim., 1937, 3, p 631-640
K.M. Myles, F.C. Mrazek, J.A. Smaga and J.L. Settle, in Advanced Battery Research and Design, U.S. ERDA Report ANL-76-8, Proceedings of Symposium and Workshop, Argonne National Laboratory, B50 (cited from [7]), 1976
E. Schürmann and H.J. Voss, Investigation of the Melting Equilibria of Mg-Li-Al Alloys, Giessereiforschung, 1981, 33, p 33
R.J. Pulham, P. Hubberstey, and P. Hemptenmacher, Solutions of Aluminum in Liquid Lithium: Contribution to the Li-Al Phase Diagram, JPE, 1994, 15, p 587-590. https://doi.org/10.1007/BF02647619
K. Kishio and J.O. Brittain, Defect Structure of β-LiAl, J. Phys. Chem. Solids, 1979, 40, p 933-940. https://doi.org/10.1016/0022-3697(79)90121-5
C.J. Wen, B.A. Boukamp, R.A. Huggins, and W. Weppner, Thermodynamic and Mass Transport Properties of “LiAl”, J. Electrochem. Soc., 1979, 126, p 2258-2266. https://doi.org/10.1149/1.2128939
E. Veleckis, Thermodynamic Investigation of the Li-Al and Li-Pb Systems by the Hydrogen Titration Method, J. Less Common Met., 1980, 73, p 49-60. https://doi.org/10.1016/0022-5088(80)90342-2
K. Amezawa, N. Yamamoto, Y. Tomii, and Y. Ito, Thermodynamic Properties and Single-Electrode Peltier Heats of a Li-Al Alloy in a LiCl-KCl Eutectic Melt, J. Electrochem. Soc., 1999, 146, p 1069-1099. https://doi.org/10.1149/1.1391722
K.F. Tebbe, H.G. von Schnering, B. Rüter, and G. Rabeneck, Li3Al2, eine neue Phase im System Li/Al/Li3Al2, a New Phase in the System Li/Al, Z. Naturforsch. B, 1973, 28, p 600-605. https://doi.org/10.1515/znb-1973-9-1010
D.A. Hansen and J.F. Smith, The Structure of Li9Al4, Acta Crystallogr. B, 1968, 24, p 913-918. https://doi.org/10.1107/S0567740868003407
N.E. Christensen, Structural Phase Stability of B2 and B32 Intermetallic Compounds, Phys. Rev. B, 1985, 32, p 207-228. https://doi.org/10.1103/PhysRevB.32.207
X.Q. Guo and R. Podloucky, Phase Stability and Bonding Characteristics of Li-rich Al-Li Intermetallic Compounds: Al2Li3 and Al4Li9, Phys. Rev. B, 1990, 42, p 10912-10923. https://doi.org/10.1103/PhysRevB.42.10912
A. Arya, G.P. Das, H.G. Salunke, and S. Banerjee, Cohesive and Electronic Properties of Ordered Li-Al Intermetallic Compounds, J. Phys. Condens. Matter, 1994, 6, p 3389-3402. https://doi.org/10.1088/0953-8984/6/18/015
M.H.F. Sluiter, Y. Watanabe, D. de Fontaine, and Y. Kawazoe, First-Principles Calculation of the Pressure Dependence of Phase Equilibria in the Al-Li System, Phys. Rev. B, 1996, 53, p 6137-6150. https://doi.org/10.1103/PhysRevB.53.6137
N.P. Yao, L.A. Herédy, and R.C. Saunders, Emf Measurements of Electrochemically Prepared Lithium-Aluminum Alloy, J. Electrochem. Soc., 1971, 118, p 1039-1042. https://doi.org/10.1149/1.2408242
W. Gąsior, A. Dębski, A. Góral, and R. Major, Enthalpy of Formation of Intermetallic Phases from Al-Li System by Solution and Direct Reaction Calorimetric Method, J. Alloys Compd., 2014, 586, p 703-708. https://doi.org/10.1016/j.jallcom.2013.10.143
Y.F. Bychkov, A.N. Rozanov, and V.B. Yakovleva, Determination of the Solubility of Metals in Lithium, Soy. J. Atomic Energy, 1961, 7, p 987-992. https://doi.org/10.1007/BF01489559
C.W. Bale, The Li-Zr (Lithium-Zirconium) System, Bull. Alloy Phase Diagr., 1987, 8, p 48-50. https://doi.org/10.1007/BF02868895
A.T. Dinsdale, SGTE Data for Pure Elements, CALPHAD, 1991, 15, p 317-425. https://doi.org/10.1016/0364-5916(91)90030-N
O. Redlich and A.T. Kister, Algebraic Representation of Thermodynamic Properties and the Classification of Solutions, J. Ind. Eng. Chem., 1948, 40, p 345-348. https://doi.org/10.1021/ie50458a036
W. Kohn and L.J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects, Phys. Rev., 1965, 140, p A1133-A1138. https://doi.org/10.1103/PhysRev.140.A1133
G. Kresse and J. Furthmueler, Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set, Phys. Rev. B, 1996, 54, p 11169-11186. https://doi.org/10.1103/PhysRevB.54.11169
Acknowledgments
The authors are grateful for the financial support from National Natural Science Foundation of China (51971189).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Long, Z., Cui, D., Hu, H. et al. Thermodynamic Modeling of the Al-Li-Zr Ternary System. J. Phase Equilib. Diffus. 41, 623–641 (2020). https://doi.org/10.1007/s11669-020-00827-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-020-00827-z