Abstract
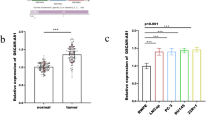
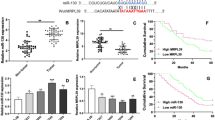
The aim of this study is to investigate the effect of lncRNA DUXAP8 on proliferation and apoptosis of ovarian cancer cells, and to explore its potential mechanism. DUXAP8 interfering and overexpressing cell lines were constructed and the cell proliferation and apoptosis were tested. Hematoxylin–eosin, TdT-mediated dUTP nick end labeling, and immunohistochemistry were used to detect the effect of DUXAP8 on the ability of tumor formation. Quantitative real-time polymerase chain reaction and western blot were used to detect the mRNA and protein expression of miR-590-5p and YAP1, respectively. Dual luciferase assay was used to determine the target relationship between DUXAP8, miR-590-5p, and YAP1. DUXAP8 interference and miR-590-5p down-regulated cell lines were further constructed. Compared with normal ovarian cells, the expression of DUXAP8 in ovarian cancer cells was significantly increased, while the expression of miR-590-5p was decreased (p < 0.05). After DUXAP8 interference, cell proliferation and colony formation were decreased, and apoptosis was increased. The results of in vivo experiment are consistent with the in vitro experiments. The expression of miR-590-5p was up-regulated and the expression of YAP1 was decreased after DUXAP8 interference. Moreover, miR590-5p inhibitor can attenuate the effect of DUXAP8 interference on ovarian cancer cells. Taken together, lncRNA DUXAP8 can regulate the proliferation and apoptosis of ovarian cancer cells, and its mechanism may be related to the regulation of YAP1 gene by targeting miR-590-5p.






Similar content being viewed by others
References
Kossaï M, Leary A, Scoazec JY, Genestie C. Ovarian cancer: a heterogeneous disease. Pathobiology. 2018;85:41–9.
Roett MA, Evans P. Ovarian cancer: an overview. Am Fam Physician. 2009;80:609–16.
Kujawa KA, Lisowska KM. Ovarian cancer - from biology to clinic. Postepy Hig Med Dosw (Online). 2015;69:1275–90.
Adams SF, Benencia F. Immunotherapy for ovarian cancer: what are the targets of the future? Future Oncol. 2015;11:1293–6.
Qi P, Du X. The long non-coding RNAs, a new cancer diagnostic and therapeutic gold mine. Mod Pathol. 2013;26:155–65.
Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, Miyano S, Mori M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6.
Struhl K. Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat Struct Mol Biol. 2007;14:103–5.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, Hung T, Argani P, Rinn JL, Wang Y, Brzoska P, Kong B, Li R, West RB, van de Vijver MJ, Sukumar S, Chang HY. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6.
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell. 2011;146:353–8.
Mitra R, Chen X, Greenawalt EJ, Maulik U, Jiang W, Zhao Z, Eischen CM. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat Commun. 2017;8:1604.
Tripathi MK, Doxtater K, Keramatnia F, Zacheaus C, Yallapu MM, Jaggi M, Chauhan SC. Role of lncRNAs in ovarian cancer: defining new biomarkers for therapeutic purposes. Drug Discov Today. 2018;23:1635–43.
Nunes FD, de Almeida FC, Tucci R, de Sousa SC. Homeobox genes: a molecular link between development and cancer. Pesqui Odontol Bras. 2003;17:94–8.
Ma HW, Xie M, Sun M, Chen TY, Jin RR, Ma TS, Chen QN, Zhang EB, He XZ, De W, Zhang ZH. The pseudogene derived long noncoding RNA DUXAP8 promotes gastric cancer cell proliferation and migration via epigenetically silencing PLEKHO1 expression. Oncotarget. 2016;8:52211–24.
Lian Y, Yang J, Lian Y, Xiao C, Hu X, Xu H. DUXAP8, a pseudogene derived lncRNA, promotes growth of pancreatic carcinoma cells by epigenetically silencing CDKN1A and KLF2. Cancer Commun (Lond). 2018;38:64.
Lian Y, Xiao C, Yan C, Chen D, Huang Q, Fan Y, Li Z, Xu H. Knockdown of pseudogene derived from lncRNA DUXAP10 inhibits cell proliferation, migration, invasion, and promotes apoptosis in pancreatic cancer. J Cell Biochem. 2018;119:3671–82.
Jiang B, Hailong S, Yuan J, Zhao H, Xia W, Zha Z, Bin W, Liu Z. Identification of oncogenic long noncoding RNA SNHG12 and DUXAP8 in human bladder cancer through a comprehensive profiling analysis. Biomed Pharmacother. 2018;108:500–7.
Huang T, Wang X, Yang X, Ji J, Wang Q, Yue X, Dong Z. Long Non-coding RNA DUXAP8 enhances Renal cell carcinoma progression via downregulating miR-126. Med Sci Monit. 2018;24:7340–7.
Xu LJ, Yu XJ, Wei B, Hui HX, Sun Y, Dai J, Chen XF. Long non-coding RNA DUXAP8 regulates proliferation and invasion of esophageal squamous cell cancer. Eur Rev Med Pharmacol Sci. 2018;22:2646–52.
Yan H, Li H, Li P, Li X, Lin J, Zhu L, Silva MA, Wang X, Wang P, Zhang Z. Long noncoding RNA MLK7-AS1 promotes ovarian cancer cells progression by modulating miR-375/YAP1 axis. J Exp Clin Cancer Res. 2018;37:237.
Lin MG, Hong YK, Zhang Y, Lin BB, He XJ. Mechanism of lncRNA DUXAP8 in promoting proliferation of bladder cancer cells by regulating PTEN. Eur Rev Med Pharmacol Sci. 2018;22:3370–7.
Ekhteraei-Tousi S, Mohammad-Soltani B, Sadeghizadeh M, Mowla SJ, Parsi S, Soleimani M. Inhibitory effect of hsa-miR-590-5p on cardiosphere-derived stem cells differentiation through downregulation of TGFB signaling. J Cell Biochem. 2015;116:179–91.
Wang J, Xu W, He Y, Xia Q, Liu S. LncRNA DUXAP8 impacts proliferation, invasion, and migration of ovarian cancer cells through regulating PTEN. Inflamm Res. 2018;67:927–36.
Zhang J, Zhou Y, Huang T, Wu F, Pan Y, Dong Y, Wang Y, Chan A, Liu L, Kwan J, Cheung A, Wong C, Lo A, Cheng A, Yu J, Lo K, Kang W, To K. FGF18, a prominent player in FGF signaling, promotes gastric tumorigenesis through autocrine manner and is negatively regulated by miR-590-5p. Oncogene. 2019;38:33–46.
Hauri S, Wepf A, van Drogen A, Varjosalo M, Tapon N, Aebersold R, Gstaiger M. Interaction proteome of human Hippo signaling: modular control of the co-activator YAP1. Mol Syst Biol. 2013;9:713.
Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, Shao DD, Schumacher SE, Weir BA, Vazquez F, Cowley GS, Root DE, Mesirov JP, Beroukhim R, Kuo CJ, Goessling W, Hahn WC. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–73.
Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP, Brabletz T. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat Commun. 2016;7:10498.
Gruber R, Panayiotou R, Nye E, Spencer-Dene B, Stamp G, Behrens A. YAP1 and TAZ Control pancreatic cancer initiation in mice by direct up-regulation of JAK-STAT3 signaling. Gastroenterology. 2016;151:526–39.
Ou C, Sun Z, Li X, Li X, Ren W, Qin Z, Zhang X, Yuan W, Wang J, Yu W, Zhang S, Peng Q, Yan Q. MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017;399:53–63.
Mou K, Ding M, Han D, Zhou Y, Mu X, Liu W, Wang L. miR-590-5p inhibits tumor growth in malignant melanoma by suppressing YAP1 expression. Oncol Rep. 2018;40:2056–66.
Acknowledgments
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interests regarding the publication of this paper.
Human and animal rights
Animal experiments were conducted following the National Institute of Health (NIH) guidelines (NIH Pub. No. 85-23, revised 1996). The experiments have been reviewed and approved by the Animal Protection and Use Committee of Jinan Maternity and Child Care Hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meng, Q., Li, Z., Pan, J. et al. Long noncoding RNA DUXAP8 regulates proliferation and apoptosis of ovarian cancer cells via targeting miR-590-5p. Human Cell 33, 1240–1251 (2020). https://doi.org/10.1007/s13577-020-00398-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00398-8