Abstract
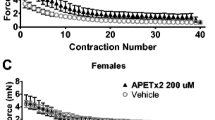
In myotonia, reduced Cl− conductance of the mutated ClC-1 channels causes hindered muscle relaxation after forceful voluntary contraction due to muscle membrane hyperexcitability. Repetitive contraction temporarily decreases myotonia, a phenomena called “warm up.” The underlying mechanism for the reduction of hyperexcitability in warm-up is currently unknown. Since potassium displacement is known to reduce excitability in, for example, muscle fatigue, we characterized the role of potassium in native myotonia congenita (MC) muscle. Muscle specimens of ADR mice (an animal model for low gCl− conductance myotonia) were exposed to increasing K+ concentrations. To characterize functional effects of potassium ion current, the muscle of ADR mice was exposed to agonists and antagonists of the big conductance Ca2+-activated K+ channel (BK) and the voltage-gated Kv7 channel. Effects were monitored by functional force and membrane potential measurements. By increasing [K+]0 to 5 mM, the warm-up phenomena started earlier and at [K+]0 7 mM only weak myotonia was detected. The increase of [K+]0 caused a sustained membrane depolarization accompanied with a reduction of myotonic bursts in ADR mice. Retigabine, a Kv7.2–Kv7.5 activator, dose-dependently reduced relaxation deficit of ADR myotonic muscle contraction and promoted the warm-up phenomena. In vitro results of this study suggest that increasing potassium conductivity via activation of voltage-gated potassium channels enhanced the warm-up phenomena, thereby offering a potential therapeutic treatment option for myotonia congenita.










Similar content being viewed by others
Change history
03 September 2020
The original article contains an error during online publication. Table 2 was included during production round and now deleted. The Original article has been corrected.
Abbreviations
- 9-AC:
-
Anthracene-9-carboxylic acid
- ADR:
-
Arrested development of rightening response
- AP:
-
Action potential
- BK channel:
-
Big conductance Ca2+-activated K+ channel
- ClC-1:
-
Skeletal muscle chloride channel type 1
- DMSO:
-
Dimethylsulfoxide
- EDL:
-
Extensor digitorum longus
- RMP:
-
Resting membrane potential
References
Adrian RH, Bryant SH (1974) On the repetitive discharge in myotonic muscle fibres. J Physiol 240:505–515
Barchi RL (1978) Muscle membrane chloride conductance and the myotonic syndromes. Contemp Clinic Neurophysiol 34:559–568
Barchi RL (2001) The pathophysiology of excitation in skeletal muscle. In: Karpati G, Hilton-Johnes D, Griggs RC (eds) Disorders of voluntary muscle, 7th edn. Cambridge University Press, Cambridge, pp 168–186
Bennetts B, Rychkov GY, Ng HL et al (2005) Cytoplasmic ATP-sensing domains regulate gating of skeletal muscle ClC-1 chloride channels. J Biol Chem 37:3452–32458
Bilmen JG, Wotton LL, Michelangeli F (2002) The mechanism of inhibition of the sarco/endoplasmic reticulum Ca2+ ATPase by paxilline. Arch Biochem Biophys 406:55–64
Birnberger KL, Klepzig M (1975) Influence of extracellar potassium and intracellar pH on myotonia. J Neurol 210:23–35
Boivin GP, Bottomley MA, Dudley ES et al (2016) Physiological, behavioral, and histological responses of male C57BL//6N mice to different CO2 chamber replacement rates. J Am Assoc Lab Anim Sci 55:451–461
Bryant SH, Morales-Aguilera A (1971) Chloride conductance in normal and myotonic muscle fibres and the action of monocarboxylic acids. J Physiol 219:383–393
Burge JA, Hanna MG (2001) Novel insights into the pathomechanisms of skeletal muscle channelopathies. Curr Neurol Neurosci Rep 12:62–69
Cannon SC (2015) Channelopathies of skeletal muscle excitability. Compr Physiol 5(2):761–790
Cannon SC (2018) Sodium channelopathies of skeletal muscle. Handb Exp Pharmacol 246:309–330
Cannon SC, Brown RH, Corey DP (1993) Theroretical reconstruction of myotonia and paralysis caused by incomplete inactivation of sodium channels. Biophys J 65:270–288
Chen MF, Niggeweg R, Laizzo PA et al (1997) Chloride conductance in mouse muscle is subject to post-transcriptional compensation of functional Cl- channel 1 gene dosage. J Physiol 504:75–81
Clausen TB (2013) Excitation induced exchange of Na+,K+, and Cl- in rat EDL muscle in vitro and vivo: physiology and pathophysiology. J Gen Physiol 141:179–192
Conte Camerino D, Tricarico D, Desaphy JF (2007) Ion channel pharmacology. Neurotherapeutics 4:184–198
De Paoli FV, Ortenblad N, Pedersen TH et al (2010) Lactate per se improves the excitability of depolarized rat skeletal muscle by reducing the Cl- conductance. J Physiol 588:4785–4794
Desaphy JF, Carbonara R, Costanza T et al (2014) Preclinical evaluation of marketed sodium channel blockers in a rat model of myotonia discloses promising antimyotonic drugs. Exp Neurol 255:96–102
Dulhunty AF (1978) The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. J Physiol 276:67–82
Dulhunty AF (1984) Heterogenity of T-tuble geometry in vertebrate skeletal muscle fibres. J Muscle Res Cell Motil 5:333–347
Dupont C, Denman KS, Hawash AA et al (2019) Treatment of myotonia congenita with retigabine in mice. Exp Neurol 315:52–59
Hawash AA, Voss AA, Rich MM (2017) Inhibiting persistent inward sodium currents prevents myotonia. Ann Neurol 82:385–395
Hoppe K, Lehmann-Horn F, Chaiklieng S et al (2013) In vitro muscle contracture investigations on the malignant hyperthermia like episodes in myotonia congenita. Acta Anaesthsiol Scand 57:1017–1023
Hoppe K, Chaiklieng S, Lehmann-Horn F et al (2018) Elevation of extracellular osmolarity improves signs of myotonia in vitro: a preclinical animal study. J Physiol 597:225–235
Iannotti FA, Panza E, Barrese V et al (2010) Expression, localization, and pharmacological role of Kv7 potassium channels in skeletal muscle proliferation, differentiation, and survival after myotoxic insults. J Pharmacol Exp Ther 332:811–820
Jurkat-Rott K, Holzherr B, Fauler M, Lehmann-Horn F (2010) Sodium channelopathies of skeletal muscle result from gain or loss of function. Pflugers Arch 460:239–248
Kristensen M, Hansen T, Juel C (2006) Membrane proteins involved in the potassium shifts during muscle activity and fatigue. Am J Phys Regul Integr Comp Phys 290:R766–R772
Kuriyama H, Korenaga S, Oda K et al (1977) Properties of muscle fiber and neuromuscular transmission in anthracene-9-carboxylic acid induced myotonia. Japan Sci Sco Press 1983:141–150
Kwiecinski H, Lehmann-Horn, Rüdel R (1988) Drugs induced myotonia in human intercostals muscle. Muscle Nerve 11:576–581
Lehmann-Horn F, Jurkatt-Rott K (2001) Channelopathies: voltage-gated sodium channels. Pharmaceut News 8:29–36
Lehmann-Horn F, Kuther G, Ricker K et al (1987) Adynamia episodica hereditaria with myotonia: a non-inactivating sodium current and the effect of extracellular pH. Muscle Nerve 10:363–374
Lehmann-Horn F, Rüdel R, Jurkat-Rott K (2004) Nondystrophic myotonias and periodic paralyses. In: Engel AG, Franzini-Armstrong C (eds) Myology, 3rd edn. McGraw-Hill, New York, pp 1257–1300
Lehmann-Horn F, Jurkat-Rott K, Rüdel R (2007) Nondystrophic myotonias and periodic paralyses. In: Rimoin DL, Connor JM, Pyeritz RE, Korf Emery BR (eds) Rimoin´s Principles and Practice of Medicine Genetics, 5th edition, vol. II. Churchill Livingstone, Elesevier, Philadelphia, pp 3024–3046
Logigian EL, Martens WB, Moxley RT IV et al (2010) Mexiletine is an effective antimyotonia treatment in myotonic dystrophy type 1. Neurology 74:1441–1448
Lossin C (2013) Nav 1.4 slow-inactivation: is it a player in the warm-up phenomenon of myotonic disorders? Muscle Nerve 47:483–487
Main MJ, Cryan JE, Dupere JRB (2000) Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol 58:258–263
McKenna MJ, Bangso J, Renaud JM (2008) Muscle K+, Na+ and Cl- disturbances and Na+-K+ pump activation: implications for fatigue. J Appl Physiol 104:288–295
Mehrke G, Brinkmeier H, Jockusch H (1988) The myotonic mouse mutant ADR: electrophysiology of the muscle fiber. Muscle Nerve 11(5):440–446
Moody CM, Chua B, Weary DM (2014) The effect of carbon dioxide flow rate on the euthanasia of laboratory mice. Lab Anim 48:298–304
Nelson CR, Debold EP, Fitts RH (2014) Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. Am J Phys Cell Phys 307:C939–C950
Novak KR, Norman J, Mitchell JR (2015) Sodium channel slow inactivation as a therapeutic target for myotonia congentia. Ann Neurol 77:320–332
Parade PT, Barchi RL (1977) On the inhibition of muscle membrane chloride conductance by aromatic carboxy acids. J Gen Physiol 69:879–896
Paterson DJ (1996) Role of potassium in the regulation of systemic physiological function during exercise. Acta Physiol 156(3):287–294
Pedersen TH, Paoli FD, Nielsen OB (2005) Increased excitability of acidified skeletal muscle: role of chloride conductance. J Gen Physiol 125:237–246
Pedersen TH, Macdonald WA, de Paoli FV et al (2009) Comparison of regulated passive membrane conductance in action of potential-firing fast and slow twitch muscle. J Gen Physiol 134:323–337
Pedersen TH, de Paoli FV, Flatman JA et al (2009) Regulation of ClC-1 and KATP channels in action potential firing fast twitch muscle fibers. J Gen Physiol 134:309–322
Satorius T, Wietzorrek G, Chaiklieng S et al (2006) BKca channel deficiency prevents low Cl- conductance myotonia, probably via reduced T-tubular K+ accumulation. International Physiology Congress, Munich
Schroeder BC, Hechenberger M, Weinreich F et al (2000) KCNQ5, a novel potassium channel broadly expressed in brain, mediates M type currents. J Biol Chem 275:24089–24095
Schwarz JR, Glassmeier G, Cooper EC et al (2006) KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol 573:17–34
Skov M, Riisager A, Fraser JA, Nielsen OB, Pedersen TH (2013) Extracellular magnesium and calcium reducemyotonia in ClC-1 inhibited rat muscle. Neuromuscul Disord 23:489–502
Statland JM, Bundy BN, Wang Y et al (2012) Consortium for Clinical Investigation of Neurologic Channelopathies, Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial. JAMA 13:1357–1365
Steinmeyer K, Klocke R, Ortland et al (1991) Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature 354:304–308
Sundberg CW, Fitts RH (2019) Bioenergetic basis of skeletal muscle fatigue. Curr Opin Physiol 10:118–127
Swift F, Stromme TA, Amundsen B et al (2006) Slow diffusion of K+ in the T-tubules of rat cardiomyocytes. J Appl Physiol 101:1170–1176
Van Beekvelt MC, Drost G, Rongen G et al (2006) Na+/K+ ATPase is not involted in the warming up phenomen in generalized myotonia. Muscle Nerve 33:514–523
Van Ernst MG, Klarenbeek S, Schot A et al (2004) Reducing chloride conductance prevents hyperkalaemia-induced loss of twitch force in rat slow-twitch muscle. J Physiol 561(1):169–181
Van Lunteren EM, Mayer M, Pollarine J (2007) Genetic CLC1-chloride channel deficiency modifies diaphragm muscle isometric contractile properties. Respir Physiol Neurobiol 155:220–226
Van Mil HG, Geukes Foppen RJ, Siegenbeek van Heukelom J (1997) The influence of bumetanide on the membrane potential of mouse skeletal muscle cells in isotonic and hypertonic media. Br J Pharmacol 120(1):39–44
Wallinga W, Meijer SL, Alberink MJ et al (1999) Modelling action potentials and membrane currents of mammalian skeletal fibres in coherence with potassium concentration changes in the T-tubular system. Eur Biophys 28:317–329
Wang GK, Russel C, Wang SY (2004) Mexiletine block of wild-type and inactivation-deficient human skeletal muscle hNav1.4Na + channels. J Physiol 554:621–633
Acknowledgments
This work is dedicated to Professor Dr Dr hc Frank Lehmann-Horn, who passed away in May 2018. His enthusiasm, helpfulness, and his bright mind are not forgotten. Part of this work were performed by Sunisa Chaiklieng and presented at the 36th European Muscle Conference in Heidelberg, Germany. Frank Lehmann-Horn and Karin Jurkat-Rott are fellows of the non-profit Hertie Foundation.
Funding
S.C. was supported by a governmental scholarship (“Land Baden-Württemberg”) for the promotion of young scientist and the German Academic Exchange Service (DAAD). We also thank the Deutsche Gesellschaft für Muskelkranke (DGM) for the grant to F.L.H. and K.J.R. for research on myotonia.
Author information
Authors and Affiliations
Contributions
Intellectual content and study design: WK, KH, FLH, SC. Collection, analysis, and interpretation of data: WK, SC, KH, FLH, KJR. Drafting the manuscript and graphical representation of data: KH, SC, WK, FLH. Critical evaluation of the manuscript: KJR, SW, WK. All authors approved the submission of this version of the manuscript. All persons designated as authors quality for authorship and all those who qualify for authorship are listed.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The original article contains an error during online publication. Table 2 was included during production round and now deleted.
Rights and permissions
About this article
Cite this article
Hoppe, K., Chaiklieng, S., Lehmann-Horn, F. et al. Preclinical pharmacological in vitro investigations on low chloride conductance myotonia: effects of potassium regulation. Pflugers Arch - Eur J Physiol 472, 1481–1494 (2020). https://doi.org/10.1007/s00424-020-02410-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02410-4