Abstract
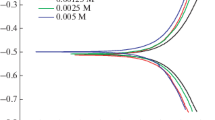
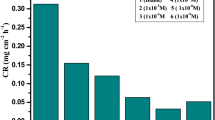
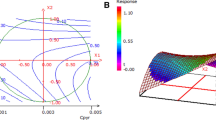
Corrosion inhibition effects of water-soluble peripheral substituted cobalt, copper and zinc metallophthalocyanines (CoPc (1), CuPc (2) and ZnPc (3)) on the copper metal in 0.1 N HCl were investigated by using gravimetric, electrochemical, SEM-EDS analysis and quantum chemical calculations. On the electrochemical investigations and gravimetric analysis the highest inhibitor efficiency was obtained with CoPc (1) at 1 × 10–2 mol/L concentration. SEM-EDS results indicated parallel results and oxygen atom content increased in the order of ZnPc (3), CuPc (2) and CoPc (1). According to the quantum chemical calculations, following corrosion inhibition efficiency ranking was obtained: CoPc (1) > CuPc (2) > ZnPc (3).














Similar content being viewed by others
REFERENCES
Antonijevic, M.M. and Petrovic, M.B., Int. J. Electrochem. Sci., 2008, vol. 3, p. 1.
Muñoz, A.I., Antón, J.G., Guiñón, J.L., and Herranz, V.P., Electrochim. Acta, 2004, vol. 50, no. 4, p. 957.
Selatnia, K.A., Sid, A., and Mosset, P., Chem. Phys. Lett., 2018, vol. 707, p. 117.
Tiana, H., Cheng, Y.F., Li, W., and Hou, B., Corros. Sci., 2015, vol. 100, p. 341.
Peña, L.F., Veyan, J.-F., Todd, M.A., Derecskei-Kovacs, A., and Chabal, Y.J., ACS Appl. Mater. Interfaces, 2010, vol. 10, no. 44, p. 38610.
Dibetsoe, M., Olasunkanmi, L.O., Fayemi, O.E., Yesudass, S., Ramaganthan, B., Bahadur, I., Adekunle, A.S., Kabanda, M.M., and Ebenso, E.E., Molecules, 2015, vol. 20, p. 15701.
Güzel, E., Atsay, A., Nalbantoglu, S., Şaki, N., Dogan, A.L., Gül, A., and Koçak, M.B., Dyes Pigm., 2013, vol. 97, p. 238.
Sukhikh, A.S., Polyakov, M.S., Klyamer, D.D., Gromilov, S.A., and Basova. T.V., J. Struct. Chem., 2017, vol. 58, p. 1039.
Güzel, E., Şaki, N., Akin, M., Nebioğlu, M., Şişman, I., Erdoğmuş, A., and Koçak, M.B., Synth. Met., 2018, vol. 245, p. 127.
Alici, E.H., Günsel, A., Akin, M., Bilgiçli, A.T., Arabaci, G., and Yarasir, M.N., J. Coord. Chem., 2018, vol. 71, no. 19, p. 3077.
Aoki, I.V., Guedes, I.C., and Maranhão, S.L.A., J. Appl. Electrochem., 2002, vol. 32, p. 915.
Li, Y., Zhao, P., and Liang, Q., Proc. Material Corrosion and Control Conference of Shandong, Qingdao, 2004, p. 196.
Nnaji, N., Nwaji, N., Mack, J., and Nyokong, T., Molecules, 2019, vol. 24, p. 207.
Günsel, A., Kocabaş, S., Bilgiçli, A.T., Güney, S., and Kandaz, M., J. Lumin., 2016, vol. 76, p. 387.
Akin, M., Nalbantoglu, S., Cuhadar, O., Uzun, D., and Saki, N., Res. Chem. Intermed., 2015, vol. 41, p. 899.
Tao, Z., Zhang, S., Li, W., and Hou, B., Ind. Eng. Chem. Res., 2011, vol. 50, p. 6082.
Becke, A.D., J. Chem. Phys., 1993, vol. 98, no.7, p. 5648.
Valle-Quitana, J.C., Dominguez-Patiño, G.F., and Gonzalez-Rodriguez, J.G., ISRN Corros., 2014, https://doi.org/10.1155/2014/945645
Chen, J. and Zhang, X., Trans. Indian Inst. Met., 2018, vol. 71, p. 1113.
Zhao, P., Liang, Q., and Li, Y., Appl. Surf. Sci., 2005, vol. 252, p. 1596.
Singh, A.K., Shukla, S.K., and Ebenso, E.E., Int. J. Electrochem. Sci., 2011, vol. 6, p. 5689.
Popova, A., Sokolova, E., Raicheva, S., Christov, M., Corros. Sci., 2003, vol. 45, p. 33.
Deyab, M.A., De Riccardis, A., and Mele, G., J. Mol. Liq., 2016, vol. 220, p. 513.
Bastidas, J.M., Pinilla, P., Cano, E., Polo, J.L., and Miguel, S., Corros. Sci., 2003, vol. 45, no. 2, p. 427.
Kaya, S., Tüzün, B., Kaya, C., Obot, I.B., J. Taiwan Inst. Chem. Eng., 2016, vol. 58, p. 528.
Kaya, S., Guo, L., Kaya, C., Tüzün, B., Obot, I.B., Touir, R., and Islam, N., J. Taiwan Inst. Chem. Eng., 2016, vol. 65, p. 522.
Obot, I.B., Kaya, S., Kaya, C., and Tüzün, B., Phys. E(Amsterdam,Neth.), 2016, vol. 80, p. 82.
Tüzün, B. and Kaya, C., J. Bio-Tribo-Corros., 2018, vol. 4, no. 4, p. 69.
Tüzün, B., and Sayin, K., Spectrochim. Acta, Part A, 2019, vol. 1141, p. 48.
Güzel, E., Günsel, A., Tüzün, B., Atmaca, G.Y., Bilgiçli, A.T., Erdoğmuş, A., and Yarasir, M.N., Polyhedron, 2019, vol. 158, p. 316.
Günsel, A., Kırbaç, E., Tüzün, B., Erdoğmuş, A., Bilgiçli, A.T., and Yaraşır, M.N., J. Mol. Struct., 2019, vol. 1180, p. 127.
Zhao, P., Liang, Q., and Li, Y., Appl. Surf. Sci., 2005, vol. 225, no. 5, p. 1596.
Lai, T.Y., Guo, J.D., Fettinger, J.C., Nagase, S., and Power, P.P., Chem. Commun., 2019, vol. 55, no. 3, p. 405.
Bentiss, F., Traisnel, M., Vezin, H., and Lagrenée, M., Corros. Sci., 2003, vol. 45, no.2, p. 371.
Khaled, K.F., Electrochim. Acta, 2003, vol. 48, no. 17, p. 2493.
Law, K.Y., Chem. Rev., 1993, vol. 93, no. 1, p. 449.
Guo, L., Safi, Z., Kaya, S., Shi, W., Tüzün, B., Altunay, N., and Kaya, C., Front. Chem., 2018, vol. 6, p. 155.
Alaoui, K., Touir, R., Galai, M., Serrar, H., Ouakki, M., Kaya, S., Tüzün, B., Boukhris, S., Ebn Touhami, M., and El Kacimi, Y., J. Bio-Tribo-Corros., 2018, vol. 4, no. 3, p. 37.
ACKNOWLEDGMENTS
This work was supported by The Research Fund of Sakarya University (Project Number: Hızdep-2019-5-19-56). This research was made possible by TUBITAK ULAKBIM, High Performance and Grid Computing Center (TR-Grid e-Infrastructure).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Akin, M., Günsel, A., Bilgiçli, A.T. et al. The Water-Soluble Peripheral Substituted Phthalocyanines as Corrosion Inhibitors for Copper in 0.1 N HCl: Gravimetric, Electrochemical, SEM-EDS, and Quantum Chemical Calculations. Prot Met Phys Chem Surf 56, 609–618 (2020). https://doi.org/10.1134/S207020512003003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S207020512003003X