Abstract
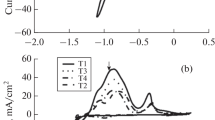
The kinetic regularities of the electrodeposition process of alloyed zinc–nickel coatings from an ammonium-chloride electrolyte solution have been studied. The effect of the addition of aminoacetic acid on the mechanism of the electrodeposition process, current efficiency, surface morphology, chemical and phase composition of the synthesized coatings has been established. The process of electrochemical deposition of alloys of Zn–Ni system from ammonium-chloride electrolyte is complicated by the diffusion transport of ions in the liquid phase of the electrolyte solution, while the kinetic stage of charge transfer is irreversible. The addition of aminoacetic acid does not change the mechanism of the electrodeposition process, however, it increases the current efficiency of the target process by approximately 6%, presumably due to a decrease in the contribution of the hydrogen evolution reaction to the overall rate of the cathodic process. The addition of glycine improves the surface morphology of the synthesized coatings by reducing the roughness and denser packing of the nuclei of the growing phase, and also increases the nickel content in the alloy by an average of 4 at %, but does not affect the phase composition of the coatings, which remain heterogeneous and consist of γ-phase of Ni2Zn11 and Zn. A quantitative assessment of the anticorrosive efficiency of electrodeposited zinc–nickel alloys is carried out depending on the presence of aminoacetic acid in the electrolyte solution. It is found that the introduction of glycine into ammonium-chloride electrolyte of deposition leads to the refinement of the corrosion potential and a noticeable decrease in the corrosion current density of the Zn, Ni-alloy coating in aqueous solution of 3.5% NaCl. This indicates an increase in the resistance of the electrochemically synthesized protective film material to corrosion damage. The optimal conditions for the electrochemical synthesis of morphologically homogeneous anticorrosive zinc–nickel coatings are selected.






Similar content being viewed by others
REFERENCES
Shestakov, M.A., Extended Abstract of Cand. Sci. (Chem.) Dissertation, Tyumen: Tyumen State Oil and Gas Univ., 2007.
Vyacheslavov, P.M., Elektroliticheskoe osazhdenie splavov (Electrolytic Deposition of Alloys), Leningrad: Mashinostroenie, 1986, p. 46.
Gaevskaya, T.V., Tsybul’skaya, L.S., and Byk, T.V., in Khimicheskie problemy sozdaniya novykh materialov i tekhnologii (Chemical Problems on Developing New Materials and Technologies), Minsk: Belarusian State Univ., 2003, issue 2, p. 100.
Vasilache, T., Gutt, S., Sandu, I., Vasilache, V., Gutt, G., Risca, M., and Sandu, A.V., Recent Pat. Corros. Sci., 2010, vol. 2, p. 1.
Lyakishev, N.P., Diagrammy sostoyaniya dvoinykh metallicheskikh sistem (State Diagrams of Binary Metal Systems), Moscow: Mashinostroenie, 2001, vol. 3, p. 670.
Lotfia, N., Aliofkhazraeia, M., Rahmanib, H., and Barati Darbanda, Gh., Prot. Met. Phys. Chem. Surf., 2018, vol. 54, no. 6, p. 1102.
Hosseini, M.G., Abdolmaleki, M., and Ashrafpoor, S., J. Appl. Electrochem., 2012, vol. 42, no. 3, p. 153.
Cai, J., Xu, J., Wang, L., Zhang, L., Zhou, H., Zhong, Y., Chen, D., Fan, H., Shao, H., Zhang, J., and Cao, C., Int. J. Hydrogen Energy, 2013, vol. 38, no. 2, p. 934.
Feng, Z., Li, Q., Zhang, J., Tang, P., Song, H., and An, M., Surf. Coat. Technol., 2015, vol. 270, p. 47.
Kondo, K., Yokoyama, M., and Shinohara, K., J. Electrochem. Soc., 1995, vol. 142, no. 7, p. 2256.
Garcia, E., Sarret, M., Müller, C., and Ortega, J.A., J. Electrochem. Soc., 2002, vol. 149, no. 5, p. 284.
Conrad, H., Corbett, J., and Goldenz, T.D., J. Electrochem. Soc., 2012, vol. 159, no. 1, p. 29.
Mosavat, S.H., Bahrololoom, M.E., and Shariat, M.H., Appl. Surf. Sci., 2011, vol. 257, no. 20, p. 8311.
Tsybulskaya, L.S., Gaevskaya, T.V., Purovskaya, O.G., and Byk, T.V., Surf. Coat. Technol., 2008, vol. 203, no. 3, p. 234.
Muresan, L.M., Stud. Univ. Babes-Bolyai, Chem., 2010, vol. 55, no. 1, p. 37.
Soares, M.E., Souza, C.A.C., and Kuri, S.E., Surf. Coat. Technol., 2006, vol. 201, no. 6, p. 2953.
Rajagopalan, S.K., Characterization of Electrodeposited Zn-Ni Alloy Coatings as a Replacement for Electrodeposited Zn and Cd Coatings, Montreal: McGill Univ., 2012.
Baldwin, K.R., Robinson, M.J., and Smith, C.J.E., Corros. Sci., 1993, vol. 35, nos. 5–8, p. 1267.
Rahsepar, M. and Bahrololoom, M.E., Corros. Sci., 2009, vol. 51, no. 11, p. 2537.
Elkhatabi, F., Sarret, M., and Müller, C., J. Electroanal. Chem., 1996, vol. 404, no. 1, p. 45.
Lin, Y. and Selman, J.R., J. Electrochem. Soc., 1993, vol. 140, no. 5, p. 1299.
Swathirajan, S., J. Electrochem. Soc., 1986, vol. 133, no. 4, p. 671.
Elkhatabi, F., Benballa, M., Sarret, M., and Müller, C., Electrochim. Acta, 1999, vol. 44, no. 10, p. 1645.
Hosseini, M.G., Ashassi-Sorkhabi, H., and Ghiasvand, H.A.Y., Surf. Coat. Technol., 2008, vol. 202, no. 13, p. 2897.
Trejo, G., Ortega, R., Meas, Y., Ozil, V.P., Chainet, E., and Nguyen, B., J. Electrochem. Soc., 1998, vol. 145, no. 12, p. 4090.
Damaskin, B.B., Petrii, O.A., and Tsirlina, G.A., Elektrokhimiya (Electrochemistry), Moscow: Khimiya, 2001.
Chouchane, S., Levesque, A., Douglade, J., Rehamnia, R., and Chopart, J.P., Surf. Coat. Technol., 2007, vol. 201, no. 14, p. 6212.
Fratesi, R. and Roventi, G., Surf. Coat. Technol., 1996, vol. 82, nos. 1–2, p. 158.
Li, G.Y., Lian, J.S., Niu, L.Y., and Jiang, Z.H., Surf. Coat. Technol., 2005, vol. 191, no. 1, p. 59.
Ghaziof, S. and Gao, W., Appl. Surf. Sci., 2014, vol. 311, p. 635.
Byk, T.V., Tsybulskaya, L.S., and Gaevskaya, T.V., Surf. Coat. Technol., 2008, vol. 202, no. 24, p. 5817.
Conrad, H.A., Corbett, J.R., and Golden, T.D., J. Electrochem. Soc., 2012, vol. 159, no. 1, p. 29.
Conde, A., Arenas, M.A., and Damborenea, J.J., Corros. Sci., 2011, vol. 53, no. 4, p. 1489.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by Sh. Galyaltdinov
Rights and permissions
About this article
Cite this article
Burlyaev, D.V., Tinaeva, A.E., Tinaeva, K.E. et al. Electrodeposition of Zinc–Nickel Coatings from Glycine-Containing Ammonium-Chloride Electrolyte. Prot Met Phys Chem Surf 56, 552–559 (2020). https://doi.org/10.1134/S2070205120030077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205120030077