Abstract
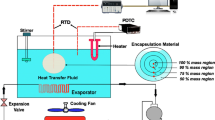
The thermal energy storage (TES) system is used to store the heat energy for longer periods and retrieve the heat energy as and when required. Experiments were conducted on the TES system with stearic acid (SA) as phase change materials (PCM) with and without iron scrap additives (IS) filled in spherical capsules. The PCM was filled in high-density polyethylene (HDPE) capsules of spherical shape. The process of charging, discharging, and the heat energy retrieved for the aforementioned PCMs were investigated and compared with various heat sources. The TES tank performance was studied with a variable/constant heat source at different flow rates, i.e. 2, 4, and 6 LPM. The results showed that the TES tank is charged to 70 °C in 204 min with 6 LPM flow rate, whereas for 2 LPM flow rate, the TES tank was charged to 70 °C in 254 min for the variable heat source. In the case of a constant heat source, to reach 70 °C, it took 54 min, 43 min, and 33 min for 2 LPM, 4 LPM, and 6 LPM flow rates, respectively. The total heat capacity of the TES tank at 70 °C was around 10,400 kJ. The output hot water at an average of 45 °C was found to be around 164 litres which means that the heat energy recovered from the TES tank was around 32%. The system with IS along PCM filled in spherical capsules was able to give 25% of hot water in extra than the same capacity of the sensible heat storage system. The results obtained reveal that heating and cooling processes were taking place at a faster rate of 13% with the addition of IS particles to the PCM when compared to pure PCM in the spherical capsules.












Similar content being viewed by others
Abbreviations
- TES:
-
Thermal energy storage
- SHS:
-
Sensible heat energy storage system
- PCM:
-
Phase change material
- LHS:
-
Latent heat energy system
- HDPE:
-
High-density polyethylene
- SA:
-
Stearic acid
- CPCM:
-
Composite phase change materials
- SNOE:
-
Silver nano-based organic ester
- HTF:
-
Heat transmission fluid
- RTD:
-
Resistance temperature detector
- EG:
-
Expanded graphite
- LPM:
-
Litres/min
- DSC:
-
Differential scanning calorimetry
- m c :
-
Mass of HTF during charging
- TCpc :
-
Specific heat of HTF during charging
- T ci :
-
Initial Temperature of HTF during charging
- T co :
-
Final Temperature of HTF during charging
- m d :
-
Mass of HTF during discharging
- C pd :
-
Specific heat of HTF during discharging
- T di :
-
Initial temperature of HTF during charging
- T do :
-
Final temperature of HTF during charging
- T o :
-
Atm. temperature
- T pcm :
-
PCM melting point temperature
- m p :
-
Mass of PCM used
- C pp :
-
Specific heat of PCM
- T pi :
-
Initial temperature of PCM
- T po :
-
Final temperature of PCM
- h sf :
-
Latent heat of PCM
References
Adref KT, Eames IW. Experiments on charging and discharging of spherical thermal (ice) storage elements. Int J Energy Res. 2002;26(11):949–64. https://doi.org/10.1002/er.816.
Assis E, Katsman L, Ziskind G, Letan R. Numerical and experimental study of melting in a spherical shell. Int J Heat Mass Transf. 2007;50(9–10):1790–804. https://doi.org/10.1016/j.ijheatmasstransfer.2006.10.007.
Barba A, Spiga M. Discharge mode for encapsulated PCMs in storage tanks. Sol Energy. 2003;74(2):141–8. https://doi.org/10.1016/S0038-092X(03)00117-8.
Chang SJ, Wi S, Jeong S-G, Kim S. Analysis on phase transition range of the pure and mixed phase change materials (PCM) using a thermostatic chamber test and differentiation. J Therm Anal Calorim. 2018;131(2):1999–2004. https://doi.org/10.1007/s10973-017-6603-y.
Keumnam C, Choi SH. Thermal characteristics of paraffin in a spherical capsule during freezing and melting processes. Int J Heat Mass Transfer. 2000;43(17):3183–96.
Chow LC, Zhong JK, Beam JE. Thermal conductivity enhancement for phase change storage media. Int Commun Heat Mass Transfer. 1996;23(1):91–100. https://doi.org/10.1016/0735-1933(95)00087-9.
Ettouney H, Hisham E-D, Amani A-A. Heat transfer during phase change of paraffin wax stored in spherical shells. J Solar Energy Eng. 2005;127(3):357–65. https://doi.org/10.1115/1.1850487.
Xiaowei F, Zhang Y, Kong W, Chen X, Wang J, Zhou C, Lei J. Synthesis and properties of bulk-biodegradable phase change materials based on polyethylene glycol for thermal energy storage. J Therm Anal Calorim. 2017;128(2):643–51. https://doi.org/10.1007/s10973-016-5959-8.
Hou P, Mao J, Liu R, Chen F, Li Y, Chang X. Improvement in thermodynamic characteristics of sodium acetate trihydrate composite phase change material with expanded graphite. J Therm Anal Calorim. 2019;137(4):1295–306. https://doi.org/10.1007/s10973-019-08061-7(0123456789(),-volV)(0123456789,-().volV).
Jegadheeswaran S, Pohekar SD, Kousksou T. Exergy based performance evaluation of latent heat thermal storage system: a review. Renew Sustain Energy Rev. 2010;14(9):2580–95. https://doi.org/10.1016/j.rser.2010.07.051.
Li Y, Yan H, Wang Q, Wang H, Huang Y. Structure and thermal properties of decanoic acid/expanded graphite composite phase change materials. J Therm Anal Calorim. 2017;128(3):1313–26. https://doi.org/10.1007/s10973-016-6068-4.
Lovera-Copa JA, Svetlana U, Nicole R, Islaman V, Franklin RM. Design of phase change materials based on salt hydrates for thermal energy storage in a range of 4–40°C. J Therm Anal Calorim. 2019;5:1–10. https://doi.org/10.1007/s10973-019-08655-1(0123456789(),-volV)(0123456789,-().volV).
Manikandan S, Selvam C, Pavan SPP, Ravita L, Kaushik DZSC, Ronggui Y. A novel technique to enhance thermal performance of a thermoelectric cooler using phase-change materials. J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08353-y(0123456789(),-vol(V0)123456789().,-volV).
Manoj Kumar P, Mylsamy K. Experimental investigation of solar water heater integrated with a nanocomposite phase change material. J Therm Anal Calorim. 2019;136(1):121–32. https://doi.org/10.1007/s10973-018-7937-9.
Nallusamy N, Sampath S, Velraj R. Study on performance of a packed bed latent heat thermal energy storage unit integrated with solar water heating system. J Zhejiang Univ Sci A. 2006;7(8):1422–30. https://doi.org/10.1631/jzus.2006.A1422.
Parameshwaran R, Jayavel R, Kalaiselvam S. Study on thermal properties of organic ester phase-change material embedded with silver nanoparticles. J Therm Anal Calorim. 2013;114(2):845–58. https://doi.org/10.1007/s10973-013-3064-9.
Radhakrishnan N, Shijo T, Sobhan CB. Characterization of thermophysical properties of nano-enhanced organic phase change materials using T-history method. J Therm Anal Calorim. 2019;5:1–14. https://doi.org/10.1007/s10973-019-08976-1.
Meenakshi Reddy R, Nallusamy N, Hariprasad T, Reddy KH, Reddy GR. Solar energy based thermal energy storage system using phase change materials. Int J Renew Energy Technol. 2011;3(1):11–23. https://doi.org/10.1504/IJRET.2012.043905.
Saleel C, Ahamed M, Abdul M, Salem A. Coconut oil as phase change material to maintain thermal comfort in passenger vehicles. J Therm Anal Calorim. 2019;136(2):629–36. https://doi.org/10.1007/s10973-018-7676-y.
Saydam V, Duan X. Dispersing different nanoparticles in paraffin wax as enhanced phase change materials. J Therm Anal Calorim. 2019;135(2):1135–44. https://doi.org/10.1007/s10973-018-7484-4(0123456789(),-volV).
Selvaraj V, Morri B, Nair LM, Krishnan H. Experimental investigation on the thermophysical properties of beryllium oxide-based nanofluid and nano-enhanced phase change material. J Therm Anal Calorim. 2019;137(5):1527–36. https://doi.org/10.1007/s10973-019-08042-w(0123456789(),-volV)(0123456789().,-volV).
Shringi V, Surendra K, Panwar NL. Experimental investigation of drying of garlic clove in solar dryer using phase change material as energy storage. J Therm Anal Calorim. 2014;118(1):533–9. https://doi.org/10.1007/s10973-014-3991-0.
Tie J, Liu X, Tie S, Zhang J, Jiang S, Tao R, Huang X. Packing and properties of composite phase change energy storage materials based on SiC nanowires and Na2SO410H2O. J Therm Anal Calorim. 2020;139(2):855–62. https://doi.org/10.1007/s10973-019-08473-5(0123456789(),-volV)(0123456789,-().volV).
Wang J, Ouyang Y, Chen G. Experimental study on charging processes of a cylindrical heat storage capsule employing multiple-phase-change materials. Int J Energy Res. 2001;25(5):439–47. https://doi.org/10.1002/er.695.
Wei S, Duan Z, Xia Y, Huang C, Ji R, Zhang H, Fen X, Sun L, Sun Y. Preparation and thermal performances of microencapsulated phase change materials with a nano-Al2O3-doped shell. J Therm Anal Calorim. 2019;138(1):233–41. https://doi.org/10.1007/s10973-019-08097-9(0123456789(),-volV)(0123456789,-().volV).
Bo W, Weixiao F, Kong B, Kai H, Zhou C, Lei J. Preparation and characterization of stearic acid/polyurethane composites as dual phase change material for thermal energy storage. J Therm Anal Calorim. 2018;132(2):907–17. https://doi.org/10.1007/s10973-018-6977-5.
Shuying W, Ma X, Peng D, Bi Y. The phase change property of lauric acid confined in carbon nanotubes as nano-encapsulated phase change materials. J Therm Anal Calorim. 2019;136(6):2353–61. https://doi.org/10.1007/s10973-018-7906-3(0123456789(),-volV)(0123456789().,-volV).
Wu SY, Wang H, Xiao S, Zhu DS. An investigation of melting/freezing characteristics of nanoparticle-enhanced phase change materials. J Therm Anal Calorim. 2012;110(3):1127–31. https://doi.org/10.1007/s10973-011-2080-x.
Zhao L, Xing Y, Liu X, Luo Y. Thermal performance of sodium acetate trihydrate based composite phase change material for thermal energy storage. Appl Therm Eng. 2018;143:172–81. https://doi.org/10.1016/j.applthermaleng.2018.07.094.
Zhou W, Wei J, Zhu J, Li K, Cheng X. Effect of Dy2O3 on thermal properties of adipic acid (AA) as phase-change materials. J Therm Anal Calorim. 2019;138(5):2999–3005. https://doi.org/10.1007/s10973-019-08315-4(012.
Arul Kumar R, Ganesh Babu B, Mohanraj M. Thermodynamic performance of forced convection solar air heaters using pin–fin absorber plate packed with latent heat storage materials. J Therm Anal Calorim. 2016;126:1657–78. https://doi.org/10.1007/s10973-016-5665-6.
Li TX, Ju-Hyuk L, Ru ZW, Yong TK. Enhancement of heat transfer for thermal energy storage application using stearic acid nanocomposite with multi-walled carbon nanotubes. Energy. 2013;55:752–61. https://doi.org/10.1016/j.energy.2013.04.010.
Prabakaran R, Kumar JP, Lal DM, Selvam C, Harish S. Constrained melting of graphene-based phase change nanocomposites inside a sphere. J Therm Anal Calorim. 2020;139(2):941–52. https://doi.org/10.1007/s10973-019-08458-4(0123456789().,-volV)(0123456789,-().volV).
Ye W-B. Enhanced latent heat thermal energy storage in the double tubes using fins. J Therm Anal Calorim. 2017;128(1):533–40. https://doi.org/10.1007/s10973-016-5870-3.
Prabakaran R, Sidney S, Lal DM, Selvam C, Harish S. Solidification of graphene-assisted phase change nanocomposites inside a sphere for cold storage applications. Energies. 2019;12(18):3473. https://doi.org/10.3390/en12183473.
Dhaidan NS, Khodadadi JM. Melting and convection of phase change materials in different shape containers: a review. Renew Sustain Energy Rev. 2015;43:449–77. https://doi.org/10.1016/j.rser.2014.11.017.
Sidney S, Dhasan ML, Harish S. Experimental investigation of freezing and melting characteristics of graphene-based phase change nanocomposite for cold thermal energy storage applications. Appl Sci. 2019;9(6):1–13. https://doi.org/10.3390/app9061099.
Kothandaraman CP. Heat and mass transfer data book. London: New Age International; 2004.
Meenakshi RR, Nallusamy N, Hemachandra RK. The effect of PCM capsule material on the thermal energy storage system performance. Renew Energy. 2014;20:1–6. https://doi.org/10.1155/2014/529280.
Moffat RJ. Using uncertainty analysis in the planning of an experiment. ASME J Fluids Eng. 1985;107:173–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tarigond, H., Reddy, R.M., Maheswari, C.U. et al. Effect of iron scrap additives in stearic acid as PCM for thermal energy storage system. J Therm Anal Calorim 141, 2497–2510 (2020). https://doi.org/10.1007/s10973-020-10117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10117-y