Abstract
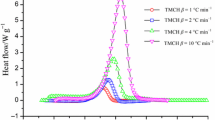
Cu etchant solutions have been widely used in etching processes because of their high etching efficiency and low cost for manufacturing electric products. Hydrogen peroxide (H2O2), a major components of Cu etchants, is known for its instability and reactivity under thermal induction, metal-ion catalysis, or pyrolysis; thus, Cu etchants have a higher risk of thermal explosion than other etchant solutions do. In this study, the exothermic reaction of the Cu etchant was initiated at approximately 70 °C, and the exothermic enthalpy (ΔH) of the first peak was measured to be approximately 274.4 J g−1 using differential scanning calorimetry 1 (DSC1). The American Society for Testing and Materials (ASTM)—Ozawa/Kissinger method, and Advanced Kinetics and Technology Solutions (AKTS)—Friedman simulation, were used to calculate the reaction kinetics. Thermal runaway and gas evolution were evaluated through vent sizing package 2 (VSP2). The pressure of the Cu etchant surged from atmospheric pressure to a maximum pressure of 225.075 psig, with a pressure increase of 168.684 psig min−1 in the VSP 2 adiabatic test. Finally, to improve the safety operation during the etching process, thermokinetic analysis was used to identify appropriate kinetic parameters for the thermal decomposition of the Cu etchant.











Similar content being viewed by others
References
Lee CY, Tsai TL. Data science framework for variable selection, metrology prediction, and process control in TFT-LCD manufacturing. Robot Comput Integr Manuf. 2019;55:76–87.
Toofan M, Toofan J. A brief review of the cleaning process for electronic device fabrication. In: Kohli R, Mittal KL, editors. Developments in surface contamination and cleaning. Oxford: William and Andrew; 2015. pp. 185–212.
Çakır O, Temel H, Kiyak M. Chemical etching of Cu-ETP copper. J Mater Process. 2005;162:275–9.
Xin F, Ma T, Wang Q. Spray etching rate development of stainless steel in the etchant for printed circuit heat exchanger channel. Energy Procedia. 2017;105:4828–35.
Zhang F, Chen M, Jia X, Xu W, Shi N. Research on the effect of resin on the thermal stability of hydrogen peroxide. Process Saf Environ Prot. 2019;126:1–6.
Gómez García MÁ, Dobrosz-Gómez I, Ojeda Toro JC. Thermal safety assessment for catalytic decomposition of hydrogen peroxide by dynamic analysis. Process Saf Environ Prot. 2017;109:46–54.
Wu RC. Hydrogen peroxide storage tank explosion in Tech-Zone corporation. Newtalk. 2019. https://newtalk.tw/news/view/2019-08-19/287736. Accessed 19 Aug 2019.
Wu D, Qian X, Liu L, Zang N. Effect of inorganic salt and organic acid on the thermal runaway of hydrogen peroxide. J Loss Prev Process Ind. 2019;57:34–40.
Ping Z, ZeYun F, Jie L, Qiang L, GuangRen Q, Ming Z. Enhancement of leaching copper by electro-oxidation from metal powders of waste printed circuit board. J Hazard Mater. 2009;166(2–3):746–50.
Liu SH, Cao CR, Lin WC, Shu CM. Experimental and numerical simulation study of the thermal hazards of four azo compounds. J Hazard Mater. 2019;365:164–77.
Wu SH, Shyu ML, Yp I, Chi JH, Shu CM. Evaluation of runaway reaction for dicumyl peroxide in a batch reactor by DSC and VSP2. J Loss Prev Process Ind. 2009;22(6):721–7.
Liu SH, Lu YM, Su C. Thermal hazard investigation and hazardous scenarios identification using thermal analysis coupled with numerical simulation for 2-(1-cyano-1-methylethyl)azocarboxamide. J Hazard Mater. 2019;384:121427.
Huang J, Jiang J, Nia L, Zhang W, Shen S, Zou M. Thermal decomposition analysis of 2,2-di-(tert-butylperoxy)butane in nonisothermal condition by DSC and GC/MS. Thermochim Acta. 2019;673:68–77.
Wang YW. Evaluation of self-heating models for peracetic acidusing calorimetry. Process Saf Environ Prot. 2018;113:122–31.
Lin YJ, Wang YW. Thermal kinetic analysis of exothermic nature and cupric ion catalysis of electronic-grade etching solutions by using calorimetry. J Loss Prev Process Ind. 2019;62:103924.
Cakir O. A Copper etching with cupric chloride and regeneration of waste etchant. J Mater Process Technol. 2006;175:63–8.
Zhu PF, Ze Y, Lin J, Liu Q, Qian GR, Zhou M. Enhancement of leaching copper by electro-oxidation from metal powders of waste printed circuit board. J Hazard Mater. 2009;166:746–50.
Shu CM, Yang YJ. Using VSP2 to separate catalytic and self-decomposition reactions for hydrogen peroxide in the presence of hydrochloric acid. Thermochim Acta. 2002;392(15):259–69.
Wu SH, Chi JH, Huang CC, Lin NK, Peng JJ, Shu CM. Thermal hazard analyses and incompatible reaction evaluation of hydrogen peroxide by DSC. J Therm Anal Calorim. 2010;102(2):563–8.
Casas JM, Alvarez F, Cifuentes L. Aqueous speciation of sulfuric acid–cupric sulfate solutions. Chem Eng Sci. 2000;55:6223–34.
Lv J, Chen L, Chen W, Gao H, Peng M. Kinetic analysis and self-accelerating decomposition temperature (SADT) of dicumyl peroxide. Thermochim Acta. 2013;571:60–3.
Abdelsalam S, Bhatti M. Anomalous reactivity of thermo-bioconvective nanofluid towards oxytactic microorganisms. Appl Math Mech. 2020;41:711–24.
Eldesoky IM, Abdelsalam SI, El-Askary WA, Ahmed MM. Concurrent development of thermal energy with magnetic field on a particle-fluid suspension through a porous conduit. BioNanoScience. 2019;9(1):186–202.
Abd-Elghany M, Klapötke TM, Elbeih A, Zeman S. Investigation of different thermal analysis techniques to determine the decomposition kinetics of ε-2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane with reduced sensitivity and its cured PBX. J Anal Appl Pyrolysis. 2017;126:267–74.
Font R, Garrido MA. Friedman and n-reaction order methods applied to pine needles andpolyurethane thermal decompositions. Thermochim Acta. 2018;660:124–33.
Acknowledgements
The authors thank the China Medical University of Taiwan (CMU-108-MF-119) for providing financial support to this study and Prof. Shu, C.M. (Process Safety and Disaster Prevention Laboratory, YunTech) for AKTS and VSP2 support to carry out our work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, YJ., Lin, ZS. & Wang, YW. Thermal runaway evaluation using DSC1, VSP2, and kinetics models on Cu etchant and its waste in high-tech etching process. J Therm Anal Calorim 144, 285–294 (2021). https://doi.org/10.1007/s10973-020-10094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10094-2