Abstract
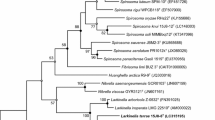
Mu Us Sandy Land in China is a very fragile ecological environment due to serious desertification. While attempting to gain insights into the biodiversity of biological soil crusts of Mu Us Sandy Land, a novel bacterial strain, SLN-3T, was isolated. It was phylogenetically placed into the genus Arthrobacter within the family Micrococcaceae based on its 16S rRNA gene sequence. The most closely related species were Arthrobacter ruber MDB1-42T (98.6%) and Arthrobacter agilis DSM 20550T (98.3%). Cells of the novel species were Gram-stain-positive, aerobic, and non-endospore-forming. The values of average nucleotide identity and the digital DNA-DNA hybridization between SLN-3T and MDB1-42T were 84.9% and 21.3%, respectively. The draft genome size of strain SLN-3T was 3.67 Mb, and its genomic G+C content was 68.1%. The predominant cellular fatty acids were anteiso-C15:0 and C17:0 anteiso. Glucose, galactose, and ribose were the whole-cell sugars. The polar lipids consisted of diphosphatidylglycerol, phosphatidylglycerol, phosphatidylinositol, glycolipid, and phospholipid. The peptidoglycan contained lysine, glutamic acid, and alanine. The predominant menaquinone was MK-9(H2). Based on the data from the chemotaxonomic, phylogenetic, and phenotypic evidence, a novel species named Arthrobacter crusticola sp. nov is proposed, whose type strain is SLN-3T (= ACCC 61595T = JCM 33723T).

Similar content being viewed by others
References
Conn HJ, Dimmick I (1947) Soil bacteria similar in morphology to Mycobacterium and Corynebacterium. J Bacteriol 54:291–303. https://doi.org/10.1146/annurev.mi.01.100147.002031
Busse HJ (2016) Review of the taxonomy of the genus Arthrobacter, emendation of the genus Arthrobacter sensu lato, proposal to reclassify selected species of the genus Arthrobacter in the novel genera Glutamicibacter gen. nov., Paeniglutamicibacter gen. nov., Pseudoglutamicibacter gen. nov., Paenarthrobacter gen. nov. and Pseudarthrobacter gen. nov., and emended description of Arthrobacter roseus. Int J Syst Evol Microbiol 66:9–37. https://doi.org/10.1099/ijsem.0.000702
Chen YG, Tang SK, Zhang YQ, Li ZY, Yi LB, Wang YX, Li WJ, Cui XL (2009) Arthrobacter halodurans sp. nov., a new halotolerant bacterium isolated from sea water. Antonie Van Leeuwenhoek 96:63–70. https://doi.org/10.1007/s10482-009-9336-5
Yan R, Fu Y, Liu D, Jiang S, Ju H, Zhao J, Wang X, Zhang J, Xiang W (2018) Arthrobacter silvisoli sp. nov., isolated from forest soil. Int J Syst Evol Microbiol 68:3892–3896. https://doi.org/10.1099/ijsem.0.003597
Yan R, Liu D, Fu Y, Zhang Y, Ju H, Zhao J, Wang X, Zhang J, Xiang W (2019) Arthrobacter celericrescens sp. nov., isolated from forest soil. Int J Syst Evol Microbiol 69:3093–3099. https://doi.org/10.1099/ijsem.0.003597
Hu QW, Chu X, Xiao M, Li CT, Yan ZF, Hozzein WN, Kim CJ, Zhi XY, Li WJ (2016) Arthrobacter deserti sp. nov., isolated from a desert soil sample. Int J Syst Evol Microbiol 66:2035–2040. https://doi.org/10.1099/ijsem.0.000986
Hoang VA, Kim YJ, Nguyen NL, Yang D (2014) Arthrobacter gyeryongensis sp. nov., isolated from soil of a Gynostemma pentaphyllum field. Int J Syst Evol Microbiol 64:420–425. https://doi.org/10.1099/ijs.0.053967-0
Lee JS, Lee KC, Pyun YR, Bae KS (2003) Arthrobacter koreensis sp. nov., a novel alkalitolerant bacterium from soil. Int J Syst Evol Microbiol 53:1277–1280. https://doi.org/10.1099/ijs.0.02492-0
Reddy GS, Aggarwal RK, Matsumoto GI, Shivaji S (2000) Arthrobacter flavus sp. nov., a psychrophilic bacterium isolated from a pond in McMurdo Dry Valley. Antarctica Int J Syst Evol Microbiol 50:1553–1561. https://doi.org/10.1099/00207713-50-4-1553
Liu Q, Xin YH, Chen XL, Liu HC, Zhou YG, Chen WX (2018) Arthrobacter ruber sp. nov. isolated from glacier ice. Int J Syst Evol Microbiol 68(5):1616–1621. https://doi.org/10.1099/ijsem.0.002719
Wang F, Gai Y, Chen M, Xiao X (2009) Arthrobacter psychrochitiniphilus sp. nov., a psychrotrophic bacterium isolated from Antarctica. Int J Syst Evol Microbiol 59:2759–2762. https://doi.org/10.1099/ijs.0.008912-0
Zhang Q, Oh M, Kim JH, Kanjanasuntree R, Konkit M, Sukhoom A, Kantachote D, Kim W (2018) Arthrobacter paludis sp. nov. isolated from a marsh. Int J Syst Evol Microbiol 68:47–51. https://doi.org/10.1099/ijsem.0.002426
İnce İA, Demirbağ Z, Katı H (2014) Arthrobacter pityocampae sp. nov. isolated from Thaumetopoea pityocampa (Lep. Thaumetopoeidae). Int J Syst Evol Microbiol 64(10):3384–3389. https://doi.org/10.1099/ijs.0.060731-0
Zhang L, Ban J (2018) Analyzing the sand-fixing effect of feldspathic sandstone from the texture characteristics. IOP Conf Ser Earth Environ Sci 108(3):032039. https://doi.org/10.1088/1755-1315/108/3/032039
Grote EE, Belnap J, Housman DC, Sparks JP (2010) Carbon exchange in biological soil crust communities under differential temperatures and soil water contents: implications for global change. Glob Change Biol 16:2763–2774. https://doi.org/10.1111/j.1365-2486.2010.02201.x
Nicholas AJ, Rebecca L, Tami LS, Niels K, Garcia-Pichel F, Benjamin PB, Richard B, Trent R (2018) Northen flux balance modeling to predict bacterial survival during pulsed-activity events. Biogeosciences 15(7):2219–2229. https://doi.org/10.5194/bg-15-2219-2018
Liu L, Hui N, Liang L, Zhang X, Sun Q, Li L (2019) Sphingomonas deserti sp. nov. isolated from Mu Us Sandy Land soil. Int J Syst Evol Microbiol 69(2):441–446. https://doi.org/10.1099/ijsem.0.003168
Liu L, Liang LX, Xu LJ, Chi M, Zhang XX, Li L (2020) Rhizobium deserti sp. nov. isolated from biological soil crusts collected at Mu Us Sandy Land. China Curr Microbiol 77:327–333. https://doi.org/10.1007/s00284-019-01831-4
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, Chichester
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. https://doi.org/10.1099/ijs.0.038075-0
Saitou N, Nei M (1987) The neighbor-joining method, a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/bf01731581
Felsenstein J (1981) Evolutionary trees from DNA sequences, a maximum likelihood approach. J Mol Evol 17:368–376. https://doi.org/10.1007/BF01734359
Rogers JS, Swofford DL (1998) A fast method for approximating maximum likelihoods of phylogenetic trees from nucleotide sequences. Syst Biol 47:77–89. https://doi.org/10.1080/106351598261049
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2014) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Bio Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
Li B, Yang X, Tan H, Ke B, He D, Ke CW, Zhang YH (2017) Vibrio parahaemolyticus O4:K8 forms a potential predominant clone in southern China as detected by whole-genome sequence analysis. Int J Food Microbiol 244:90–95. https://doi.org/10.1016/j.ijfoodmicro.2017.01.001
Tatusova T, Dicuccio M, Badretdin A, Chetvernin V, Nawrocki EP, Zaslavsky L, Lomsadze A, Pruitt KD, Borodovsky M, Ostell J (2016) NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res 44:6614–6624. https://doi.org/10.1093/nar/gkw569
Richter M, Rosselló-Móra R, Glöckner FO, Peplies J (2016) JSpeciesWS: a web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 32(6):929–931. https://doi.org/10.1093/bioinformatics/btv681
Meier-Kolthoff JP, Auch AF, Klenk HP, Göker M (2013) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinformatics 14:60–73. https://doi.org/10.1186/1471-2105-14-60
Komagata K, Suzuki K (1987) Lipid and cell-wall analysis in bacterial systematics. Methods Microbiol 19:161–207. https://doi.org/10.1016/S0580-9517(08)70410-0
Minnikin DE, O’Donnell AG, Goodfellow M, Alderson G, Athalye M, Schaal A, Parlett JH (1984) An integrated procedure for the extraction of isoprenoid quinones and polar lipids. J Microbiol Methods 2:233–241. https://doi.org/10.1016/0167-7012(84)90018-6
Lechevalier MP, Lechevalier HA (1980) The chemotaxonomy of actinomycetes. In: Actinomycete Taxonomy (Special Publication no. 6). Arlington: society for industrial microbiology, pp 227–291.
Sun HM, Zhang T, Wei YZ, Liu HY, Yu LY, Zhang YQ (2015) Tenggerimyces mesophilus gen. nov., sp. nov., a member of the family Nocardioidaceae. Int J Syst Evol Microbiol 65:3359–3364. https://doi.org/10.1099/ijsem.0.000421
Dastager SG, Qin L, Tang SK, Krishnamurthi S, Lee JC, Li WJ (2014) Arthrobacter enclensis sp. nov., isolated from sediment sample. Arch Microbiol 196:775–782. https://doi.org/10.1007/s00203-014-1016-9
Hasegawa T, Takizawa M, Tanida S (1983) A rapid analysis for chemical grouping of aerobic actinomycetes. J Gen Appl Microbiol 29:319–322
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids, MIDI Technical Note 101. MIDI Inc, Newark, DE
Richter M, Rosselló-Móra R (2009) Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci 106:19126–19131. https://doi.org/10.1073/pnas.090641210
Rosselló-Móra R, Trujillo ME, Sutcliffe IC (2017) Introducing a digital protologue: a timely move towards a database-driven systematics of archaea and bacteria. Antonie Van Leeuwenhoek 110:455–545. https://doi.org/10.1016/j.syapm.2017.02.001
Funding
This work was supported by the State Key Research and Development Program of China (2016YFC0500801). National Natural Science Foundation of China (31800522).
Author information
Authors and Affiliations
Contributions
LL conceived the study, participated in its design, and coordinated and drafted the manuscript; LL, SH, LX, MC and SS participated in the design and coordination of the study and performed the measurement; XZ and LL conceived the study, participated in its design, and coordinated and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
No specific ethical or institutional permits were required to conduct sampling, and the experimental studies did not involve endangered or protected species.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, L., Liang, L., He, S. et al. Arthrobacter crusticola sp. nov., Isolated from Biological Soil Crusts in the Mu Us Sandy Land, China. Curr Microbiol 77, 2042–2048 (2020). https://doi.org/10.1007/s00284-020-02070-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02070-8