Treatment of Cheese Whey Wastewater Using an Expanded Granular Sludge Bed (EGSB) Bioreactor with Biomethane Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cheese Whey Wastewater (CWW)
2.2. AGS Acclimatization
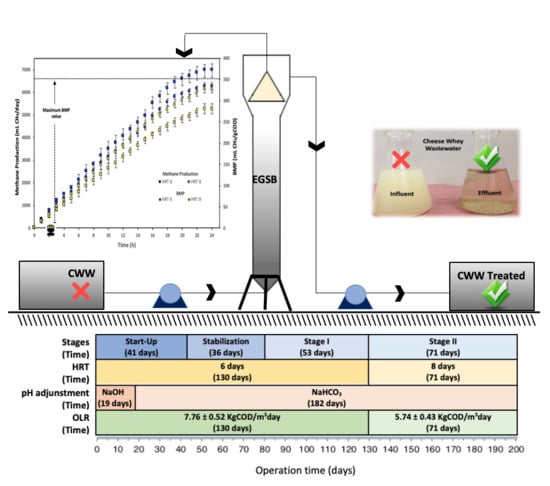
2.3. Experimental Set Up
2.4. Reactor Operation
2.5. Analytical Methods
3. Results and Discussion
3.1. CWW Characterization
3.2. AGS Acclimatization
3.3. EGSB Bioreactor Operation Analysis
3.4. Biomethane Production
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguilar, C. Tipología de las cadenas industriales y la calidad de la leche en los Altos de Jalisco. Temas Ciencia Tecnología 2003, 7, 13–23. [Google Scholar]
- Castañeda-Martínez, T.; Boucher, F.; Sánchez-Vera, E.; Espinoza-Ortega, A. La concentración de agroindustrias rurales de producción de quesos en el noroeste del Estado de México: Un estudio de caracterización. Estudios Sociales 2008, 17, 75–109. [Google Scholar]
- Bello, J.M.L.; Lizeldi, B.V.; González, E.V.; Manzo, A.S.; Nochebuena, X.P.; Quiñones-Ramírez, E.I.; Vázquez-Salinas, C. Productos Lácteos: La ruta de la metamorfosis. Rev. Digit. Univ. 2004, 5, 1–14. [Google Scholar]
- Varnam, A.H.; Sutherland, J.P. Leche y Productos Lácteos, Ciencia y Tecnología de los Alimentos; Acribia: Zaragoza, España, 1995. [Google Scholar]
- Cruz-Salomón, A. Design and Evaluation of EGSB Bioreactors for the Treatment of Agro-Industrial Wastewaters of the State of Chiapas. Ph.D. Thesis, UNICACH, Chiapas, Mexico, 2018. [Google Scholar]
- Siciliano, M. Estudio de la Vida útil de Queso Crema Utilizando Microbiología Predictiva. Master’s Thesis, Universidad Tecnológica Nacional, Buenos Aires, Argentina, 2010. [Google Scholar]
- Gandolfo, C. Tecnología de la Elaboración de la Nata: Procesos Comunes. Available online: https://www.agroterra.com/blog/profesionales/tecnologia-de-la-elaboracion-de-la-nata-procesos-comunes/75849/ (accessed on 10 May 2020).
- Banks, W. Dairy products: Technology. J. Soc. Dairy Technol. 1993, 46, 83–86. [Google Scholar] [CrossRef]
- Sinigaglia, M.; Bevilacqua, A.M.; Corbo, M.R.; Pati, S.; Del Nobile, M.A. Use of active compounds for prolonging the shelf life of mozzarella cheese. Int. Dairy J. 2007, 18, 624–630. [Google Scholar] [CrossRef]
- Poncelet. Poncelet: La Enciclopedia del Queso. Available online: www.poncelet.es (accessed on 10 May 2020).
- Cruz-Salomón, A.; Ríos-Valdovinos, E.; Pola-Albores, F.; Meza-Gordillo, R.; Lagunas-Rivera, S.; Ruíz-Valdiviezo, V.M. Anaerobic treatment of agro-industrial wastewater for COD removal in expanded granular sludge bed bioreactor. Biofuel Res. J. 2017, 16, 715–720. [Google Scholar] [CrossRef]
- Ergúder, T.H.; Tezel, U.; Gúven, E.; Demirer, G.N. Anaerobic biotransformation and methane generation potential of cheese whey in batch and UASB reactors. Waste Manag. 2001, 21, 643–650. [Google Scholar] [CrossRef]
- Mawson, A.J. Bioconversion for whey utilization and waste abatement. Biores. Technol. 1994, 47, 195–203. [Google Scholar] [CrossRef]
- Gelegenis, J.; Georgakakis, D.; Angelidaki, I.; Mavris, V. Optimization of biogas production by co-digesting whey with diluted poultry manure. Renew. Energy 2007, 32, 2147–2160. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kennedy, J.F.; Gandhi, D.N.; Bunko, K. Bioutilisation of whey for lactic acid production. Food Chem. 2007, 105, 1–14. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Janczukowicz, W.; Zielinski, M.; Dębowski, M. Biodegradability evaluation of dairy effluents originated in selected sections of dairy production. Bioresour. Technol. 2008, 99, 4199–4205. [Google Scholar] [CrossRef] [PubMed]
- Diario Oficial de la Federación (DOF). Programa Nacional Hídrico 2014–2018. Available online: http://www.dof.gob.mx/nota_detalle.php?codigo=5339732&fecha=08/04/2014 (accessed on 19 May 2020).
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources. An Introduction; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Borja, R.; Rincón, B. Biogas Production. Ref. Modul. Life Sci. 2017, 1–24. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, C.; Martínez-Torres, E.J.; Morán-Palao, A.; Gómez-Barrios, X. Procesos biológicos para el tratamiento de lactosuero con producción de biogás e hidrógeno. Revisión Bibliográfica Revion 2016, 29, 47–62. [Google Scholar] [CrossRef] [Green Version]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic treatment of dairy wastewaters: A review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Cruz-Salomón, A.; Ríos-Valdovinos, E.; Pola-Albores, F.; Lagunas-Rivera, S.; Meza-Gordillo, R.; Ruíz-Valdiviezo, V.M.; Cruz-Salomón, K.C. Expanded granular sludge bed bioreactor in wastewater treatment. Glob. J. Environ. Sci. Manag. 2019, 5, 119–138. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 21th ed.; Association of Analytical Chemists: Arlington, VA, USA, 2019. [Google Scholar]
- Walkley, A.; Black, I. An examination of the Degtjareff method for determining organic carbon in soils: Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 2006, 63, 251–263. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Buitrón, G. Variation of the microbial activity during the acclimation phase of an SBR system degrading 4-chlorophenol. Water Sci. Technol. 2004, 5, 251–258. [Google Scholar] [CrossRef]
- Ramos-Vaquerizo, F.; Cruz-Salomón, A.; Ríos-Valdovinos, E.; Pola- Albores, F.; Lagunas-Rivera, S.; Ruíz-Valdiviezo, V.M.; Simuta-Champo, R.; Moreira-Acosta, J. Anaerobic treatment of vinasse from sugarcane ethanol production in expanded granular sludge bed bioreactor. J. Chem. Eng. Process. Technol. 2018, 9, 375. [Google Scholar]
- Normas Mexicanas (NMX-AA-045-SCFI-2001). Análisis de Agua-Determinación de Color Platino Cobalto en Aguas Naturales, Residuales y Residuales Tratadas. Available online: https://www.gob.mx/cms/uploads/attachment/file/166781/NMX-AA-045-SCFI-2001.pdf (accessed on 19 May 2020).
- Norma Mexicana (NMX-AA-026-SCFI-2010). Análisis de Agua-Determinación de Nitrógeno Total Kjeldahl en Aguas Naturales, Residuales y Residuales Tratadas. Available online: https://www.gob.mx/cms/uploads/attachment/file/166772/NMX-AA-026-SCFI-2010.pdf (accessed on 19 May 2020).
- Cruz-Salomón, A.; Ríos-Valdovinos, E.; Pola-Albores, F.; Lagunas-Rivera, S.; Meza-Gordillo, R.; Ruíz-Valdiviezo, V.M. Evaluation of hydraulic retention time on treatment of coffee processing wastewater (CPWW) in EGSB bioreactor. Sustainability 2018, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Salomón, A.; Meza-Gordillo, R.; Rosales-Quintero, A.; Ventura-Canseco, C.; Lagunas-Rivera, S.; Carrasco-Cervantes, J. Biogas production from a native beverage vinasse using a modified UASB bioreactor. Fuel 2017, 198, 170–174. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guideline for Discharge of Industrial Effluent Characteristics; WHO: Geneva, Switzerland, 1995; Volume 3, pp. 231–236. [Google Scholar]
- Official Mexican Environmental Regulations. NOM-001- SEMARNAT-1996; Comisión Nacional del Agua: Ciudad de México, México, 2015.
- Elliott, M.; Jonge, V.N. The management of nutrients and potential eutrophication in estuaries and other restricted water bodies. Hydrobiologia 2002, 475, 513–524. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Fenton-like application to pretreated cheese whey wastewater. J. Environ. Manag. 2013, 129, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Osorio-González, C.S.; Sandoval-Salas, F.; Hernández-Rosas, F.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C.; Ávalos de la Cruz, D.A. Potential for the use of cheese whey in Mexico. Agroproductividad 2018, 11, 101–106. [Google Scholar]
- Eze, V.C.; Onwuakor, C.E.; Orok, F.E. Microbiological and Physicochemical Characteristics of Soil Contaminated With Used Petroleum Products in Umuahia, Abia State, Nigeria. J. Appl. Environ. Microbiol. 2014, 2, 281–286. [Google Scholar]
- Lükewille, A.; Jeffries, D.; Johannessen, M.; Raddum, G.; Stoddard, J.; Traaen, T. Transboundary Air Pollution. International Cooperative Programmed on Assessment and Monitoring of Acidification of Rivers and Lakes. The Nine-Year Report: Acidification of Surface Water in Europe and North America–Long-Term Developments (1980s and 1990s); NIVA-Report n° 3637–97:168; ICP Waters: Oslo, Norway, 2003. [Google Scholar]
- Vázquez-Esnoval, C.O.; Pinto-Ruiz, R.; Rodríguez-Hernández, R.; Carmona de la Torre, J.; Gómez de Jesús, A. Use, production and nutritional quality of whey milk in the central region of Chiapas. Avances Investigación Agropecuaria 2017, 21, 65–75. [Google Scholar]
- Aelion, C.M.; Dobbins, D.C.; Pfaender, F.K. Adaptation of aquifer microbial communities to the biodegradation of xenobiotic compounds: Influence substrate concentration and preexposure. Environ. Toxicol. Chem. 1989, 8, 75–86. [Google Scholar] [CrossRef]
- Buitrón, G.; Moreno, J. Modeling of the acclimation/deacclimation processes of a mixed culture degrading 4-chlorophenol. Water Sci. Technol. 2004, 49, 79–86. [Google Scholar] [CrossRef]
- Wiggings, B.A.; Jones, S.H.; Alexander, M.A. Explanations for the acclimation period preceding the mineralization of organic chemicals in aquatic environments. App. Environ. Microbiol. 1987, 53, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Gavala, H.N.; Angelidaki, I.; Birgitte, K. Kinetics and Modeling of Anaerobic Digestion Process. Adv. Biochem. Eng. Biotechnol. 2003, 81, 58–93. [Google Scholar]
- Pérez, A.; Torres, P. Alkalinity indices for control of anaerobic treatment of readily acidifiable wastewaters. Ingeniería Competitividad 2008, 10, 41–45. [Google Scholar]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Malaspina, F.; Stante, L.; Cellamare, C.M.; Tilche, A. Cheese whey and cheese factory wastewater treatment with a biological anaerobic–aerobic process. Water Sci. Technol. 1995, 32, 59–72. [Google Scholar] [CrossRef]
- Rojas, O. La Alcalinidad Como Parámetro de Control de los Ácidos Grasos Volátiles en Digestores UASB; Curso Sobre Tratamiento Anaerobio de Aguas Residuales, Programa de Posgrado en Ingeniería Sanitaria y Ambiental, Universidad del Valle: Cali, Colombia, 2004; pp. 101–105. [Google Scholar]
- Rodgers, M.; Xin-Min, Z.; Dolan, B. Mixing characteristics and whey wastewater treatment of a novel moving anaerobic biofilm reactor. J. Environ. Sci. Health Part A Toxic/Hazard Subst. Environ. Eng. 2004, 39, 2183–2193. [Google Scholar] [CrossRef]
- Borja, R.; Martin, A.; Luque, M.; Alonso, V. Kinetics of methane production from wine distillery wastewater in an immobilised cell bioreactor using sepiolite as support medium. Resour. Conserv. Recycl. 1994, 10, 317–327. [Google Scholar] [CrossRef]
- Van den Berg, L. High rate reactors for methane production. In Biotechnology and Renewable Energy; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 1986; pp. 268–275. [Google Scholar]
- Michaud, S.; Berneta, N.; Buffiére, P.; Roustan, M.; Moletta, R. Methane yield as a monitoring parameter for the start-up of anaerobic fixed film reactors. Water Res. 2002, 36, 1385–1391. [Google Scholar] [CrossRef]
- Jennett, J.C.; Dennis, N.D. Anaerobic filter treatment of pharmaceutical waste. J. Water Pollut. Control Fed. 1975, 45, 104–121. [Google Scholar]
- Labatut, R.A.; Largus, T.A.; Norman, R.S. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Mainardis, M.; Cabbai, V.; Zannier, G.; Visintini, D.; Goi, D. Characterization and BMP Tests of Liquid Substrates for High-rate Anaerobic Digestion Chem. Biochem. Eng. Q. 2017, 31, 509–518. [Google Scholar] [CrossRef]
- Salunkhe, D.B.; Rai, R.K.; Borkar, R.P. Biogas technology. Int. J. Eng. Sci. Technol. 2012, 5, 4934–4940. [Google Scholar]







| Examination and Determination | Frequency | Reference |
|---|---|---|
| Influent and Effluent | ||
| pH | D | (Method 4500-H+–B) [23] |
| Temperature | D | (Method 2550–B) [23] |
| COD | T | (Method 5220–D) [23] |
| BOD5 | M | (Method 5210–B) [23] |
| TS | W | (Method 2540–B) [23] |
| Color | M | (NMX-AA-045-SCFI-2001) [29] |
| Turbidity | M | (Method 2130–B) [23] |
| TN | M | (NMX-AA-026-SCFI-2010) [30] |
| Buffer Index (BI) | T | (Titrimetric Method) [11] |
| COD removal efficiency | T | Mathematical Equation [31] |
| Biomethane | ||
| Biomethane Production | T | (Volumetric Method) [32] |
| Biochemical methane potential (BMP) | T | (Volumetric Method) [28] |
| Biomethane production rate (MPR) | T | (Volumetric Method) [5] |
| Parameter | Values | Permissible Limits | |
|---|---|---|---|
| WHO | Mexico * | ||
| pH | 4.33 ± 0.21 | 6.5–8.5 | 5–10 |
| Moisture (%) | 95.23 ± 1.35 | - | - |
| Ash (%) | 3.75 ± 0.3 | - | - |
| Density (g/mL) | 1.137 ± 0.02 | - | - |
| Viscosity (mPa·s) | 0.986 ± 0.01 | - | - |
| Color (Pt-Co) | 9366 ± 328 | - | - |
| Turbidity (NTU) | 426 ± 24 | 5 | - |
| EC (mS/cm) | 8.5 ± 0.3 | - | - |
| Floating matter | Present | - | Absent |
| Settleable solids (mL/L) | 13 ± 1 | - | 2 |
| TS (mg/L) | 47,643 ± 1358 | 650 | - |
| TVS (mg/L) | 42,873 ± 3433 | - | - |
| TSS (mg/L) | - | 200 | 12–5 |
| Acidity (mg CaCO3/L) | 3379 ± 610 | - | - |
| Alkalinity (mg CaCO3/L) | ND | - | - |
| FOG (mg/L) | 5495 ± 480 | - | - |
| COD (mgO2/L) | 91,600 ± 7950 | 300 | - |
| BOD5 (mgO2/L) | 90,083 ± 6742 | 100 | 150 |
| TOC (mg/L) | 33,400 ± 2742 | - | - |
| TP (mg/L) | 707 ± 91 | - | 30 |
| TN (mg/L) | 2200 ± 185 | - | 60 |
| Sulfates (mg/L) | 17 ± 0.84 | 250 | - |
| BI | 0.98 ± 0.01 | - | - |
| CI | 5388 ± 259 | - | - |
| FWI | 0.056 ± 0.002 | - | - |
| C/N | 15/1 | - | - |
| Parameters | Influent | Effluent | E (%) | Permissible Limits | |||
|---|---|---|---|---|---|---|---|
| Stage I | Stage II | Stage I | Stage II | WHO | Mexico * | ||
| COD (mg O2/L) | 46,348 ± 3200 | 4548 ± 346 | 3468 ± 439 | 90 | 92 | 300 | - |
| BOD5 (mg O2/L) | 45,421 ± 3036 | 4080 ± 264 | 2971 ± 214 | 91 | 93 | 100 | 150 |
| TS (mg/L) | 19,558 ± 1350 | 5661 ± 553 | 4843 ± 481 | 71 | 75 | 650 | - |
| TN (mg/L) | 1090.8 | 138 ± 22 | 119 ± 18 | 87 | 89 | - | 60 |
| Color (Pt-Co) | 4683 | 2547 ± 145 | 2389 ± 128 | 46 | 49 | - | - |
| Turbidity (NTU) | 277 | 121 ± 27 | 116 ± 19 | 56 | 58 | 5 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Salomón, A.; Ríos-Valdovinos, E.; Pola-Albores, F.; Lagunas-Rivera, S.; Cruz-Rodríguez, R.I.; Cruz-Salomón, K.d.C.; Hernández-Méndez, J.M.E.; Domínguez-Espinosa, M.E. Treatment of Cheese Whey Wastewater Using an Expanded Granular Sludge Bed (EGSB) Bioreactor with Biomethane Production. Processes 2020, 8, 931. https://doi.org/10.3390/pr8080931
Cruz-Salomón A, Ríos-Valdovinos E, Pola-Albores F, Lagunas-Rivera S, Cruz-Rodríguez RI, Cruz-Salomón KdC, Hernández-Méndez JME, Domínguez-Espinosa ME. Treatment of Cheese Whey Wastewater Using an Expanded Granular Sludge Bed (EGSB) Bioreactor with Biomethane Production. Processes. 2020; 8(8):931. https://doi.org/10.3390/pr8080931
Chicago/Turabian StyleCruz-Salomón, Abumalé, Edna Ríos-Valdovinos, Francisco Pola-Albores, Selene Lagunas-Rivera, Rosa Isela Cruz-Rodríguez, Kelly del Carmen Cruz-Salomón, Jesús Mauricio Ernesto Hernández-Méndez, and María Emperatriz Domínguez-Espinosa. 2020. "Treatment of Cheese Whey Wastewater Using an Expanded Granular Sludge Bed (EGSB) Bioreactor with Biomethane Production" Processes 8, no. 8: 931. https://doi.org/10.3390/pr8080931