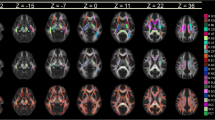
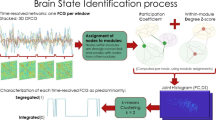
Abstract
Traumatic brain injury (TBI) and Alzheimer’s disease (AD) are prominent neurological conditions whose neural and cognitive commonalities are poorly understood. The extent of TBI-related neurophysiological abnormalities has been hypothesized to reflect AD-like neurodegeneration because TBI can increase vulnerability to AD. However, it remains challenging to prognosticate AD risk partly because the functional relationship between acute posttraumatic sequelae and chronic AD-like degradation remains elusive. Here, functional magnetic resonance imaging (fMRI), network theory, and machine learning (ML) are leveraged to study the extent to which geriatric mild TBI (mTBI) can lead to AD-like alteration of resting-state activity in the default mode network (DMN). This network is found to contain modules whose extent of AD-like, posttraumatic degradation can be accurately prognosticated based on the acute cognitive deficits of geriatric mTBI patients with cerebral microbleeds. Aside from establishing a predictive physiological association between geriatric mTBI, cognitive impairment, and AD-like functional degradation, these findings advance the goal of acutely forecasting mTBI patients’ chronic deviations from normality along AD-like functional trajectories. The association of geriatric mTBI with AD-like changes in functional brain connectivity as early as ~6 months post-injury carries substantial implications for public health because TBI has relatively high prevalence in the elderly.




Similar content being viewed by others
Data availability
MRI data acquired from HC and AD participants are publicly available from the ADNI database (http://adni.loni.usc.edu). For TBI participants, primary data generated during and/or analyzed during the current study are available subject to a data transfer agreement. At the request of some participants, their written permission is additionally required in a limited number of cases.
References
de Freitas Cardoso MG, Faleiro RM, de Paula JJ, Kummer A, Caramelli P, Teixeira AL, et al. Cognitive impairment following acute mild traumatic brain injury. Front Neurol. 2019;10:198.
Irimia A, Goh SY, Torgerson CM, Vespa P, Van Horn JD. Structural and connectomic neuroimaging for the personalized study of longitudinal alterations in cortical shape, thickness and connectivity after traumatic brain injury. J Neurosurg Sci. 2014;58(3):129–44.
Tripodis Y, Alosco ML, Zirogiannis N, Gavett BE, Chaisson C, Martin B, et al. The effect of traumatic brain injury history with loss of consciousness on rate of cognitive decline among older adults with normal cognition and Alzheimer’s disease dementia. J Alzheimers Dis. 2017;59(1):251–63. https://doi.org/10.3233/Jad-160585.
Griesbach GS, Masel BE, Helvie RE, Ashley MJ. The impact of traumatic brain injury on later life: effects on normal aging and neurodegenerative diseases. J Neurotrauma. 2018;35(1):17–24. https://doi.org/10.1089/neu.2017.5103.
Gardner RC, Dams-O'Connor K, Morrissey MR, Manley GT. Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J Neurotrauma. 2018;35:889–906. https://doi.org/10.1089/neu.2017.5371.
Van Horn JD, Irimia A, Torgerson CM, Bhattrai A, Jacokes Z, Vespa PM. Mild cognitive impairment and structural brain abnormalities in a sexagenarian with a history of childhood traumatic brain injury. J Neurosci Res. 2018;96(4):652–60. https://doi.org/10.1002/jnr.24084.
Faden AI, Loane DJ. Chronic Neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics. 2015;12(1):143–50. https://doi.org/10.1007/s13311-014-0319-5.
Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014;71(12):1490–7. https://doi.org/10.1001/jamaneurol.2014.2668.
Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci. 2015;66:75–80. https://doi.org/10.1016/j.mcn.2015.03.001.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47. https://doi.org/10.1146/annurev-neuro-071013-014030.
Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, et al. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage. 2012;59(1):511–8. https://doi.org/10.1016/j.neuroimage.2011.07.081.
Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100(24):14504–9. https://doi.org/10.1073/pnas.2235925100.
Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Hum Brain Mapp. 2011;32(11):1825–35. https://doi.org/10.1002/hbm.21151.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–65. https://doi.org/10.1152/jn.00338.2011.
Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, et al. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012;265(3):882–92. https://doi.org/10.1148/radiol.12120748.
Irimia A, Van Horn JD, Vespa PM. Cerebral microhemorrhages due to traumatic brain injury and their effects on the aging human brain. Neurobiol Aging. 2018;66:158–64.
Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74(3):201–9. https://doi.org/10.1212/WNL.0b013e3181cb3e25.
Fan D, Chaudhari NN, Rostowsky KA, Calvillo M, Lee SK, Chowdhury NF, et al. Post-traumatic cerebral microhemorrhages and their effects upon white matter connectivity in the aging human brain. In: Conference Proceedings of the IEEE Engineering in Medicine and Biology Society. Venice: IEEE; 2019. p. 198–203.
Jack CR Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27(4):685–91. https://doi.org/10.1002/jmri.21049.
Khazaee A, Ebrahimzadeh A, Babajani-Feremi A. Identifying patients with Alzheimer’s disease using resting-state fMRI and graph theory. Clin Neurophysiol. 2015;126(11):2132–41. https://doi.org/10.1016/j.clinph.2015.02.060.
Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. https://doi.org/10.1016/j.neuroimage.2010.06.010.
Wellek S. A new approach to equivalence assessment in standard comparative bioavailability trials by means of the Mann-Whitney statistic. Biometrical J. 1996;38(6):695–710. https://doi.org/10.1002/bimj.4710380608.
Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med. 2011;26(2):192–6. https://doi.org/10.1007/s11606-010-1513-8.
Hoffelder T, Gossl R, Wellek S. Multivariate equivalence tests for use in pharmaceutical development. J Biopharm Stat. 2015;25(3):417–37. https://doi.org/10.1080/10543406.2014.920344.
Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech-Theory E. 2008;2008. https://doi.org/10.1088/1742-5468/2008/10/P10008.
Cuthill E, McKee J. Reducing the bandwidth of sparse symmetric matrices. New York: Twenty-fourth National Conference of the ACM; 1969.
Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26(1):288–303. https://doi.org/10.1093/cercor/bhu239.
Irimia A, Van Horn JD. Scale-dependent variability and quantitative regimes in graph-theoretic representations of human cortical networks. Brain Connect. 2016;6(2):152–63. https://doi.org/10.1089/brain.2015.0360.
Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26(3):297–302. https://doi.org/10.2307/1932409.
Rencher AC. Methods of multivariate analysis. New York: John Wiley & Sons, Inc.; 2002.
Matthews BW. Comparison of the predicted and observed secondary structure of T4 phage lysozyme. Biochim Biophys Acta. 1975;405(2):442–51. https://doi.org/10.1016/0005-2795(75)90109-9.
Irimia A, Van Horn JD. Functional neuroimaging of traumatic brain injury: advances and clinical utility. Neuropsychiatr Dis Treat. 2015;11:2355–65. https://doi.org/10.2147/NDT.S79174.
Zverova M. Alzheimer’s disease and blood-based biomarkers - potential contexts of use. Neuropsychiatr Dis Treat. 2018;14:1877–82. https://doi.org/10.2147/NDT.S172285.
Irimia A, Torgerson CM, Goh SY, Van Horn JD. Statistical estimation of physiological brain age as a descriptor of senescence rate during adulthood. Brain Imaging Behav. 2015;9(4):678–89. https://doi.org/10.1007/s11682-014-9321-0.
Anderson CV, Bigler ED, Blatter DD. Frontal lobe lesions, diffuse damage, and neuropsychological functioning in traumatic brain-injured patients. J Clin Exp Neuropsychol. 1995;17(6):900–8. https://doi.org/10.1080/01688639508402438.
Harris TC, de Rooij R, Kuhl E. The shrinking brain: cerebral atrophy following traumatic brain injury. Ann Biomed Eng. 2019;47(9):1941–59. https://doi.org/10.1007/s10439-018-02148-2.
Anderson V, Jacobs R, Anderson PJ. Executive functions and the frontal lobes: A lifespan perspective. New York NY and Oxford UK: Taylor and Francis. 2008;XXVII–XXXIII. https://brainmaster.com/software/pubs/books/Executive_Functions_and_the_Frontal_Lobes.pdf
Venkatesan UM, Dennis NA, Hillary FG. Chronology and chronicity of altered resting-state functional connectivity after traumatic brain injury. J Neurotrauma. 2015;32(4):252–64. https://doi.org/10.1089/neu.2013.3318.
Iraji A, Benson RR, Welch RD, O'Neil BJ, Woodard JL, Ayaz SI, et al. Resting state functional connectivity in mild traumatic brain injury at the acute stage: independent component and seed-based analyses. J Neurotrauma. 2015;32(14):1031–45. https://doi.org/10.1089/neu.2014.3610.
Pasquini L, Scherr M, Tahmasian M, Meng C, Myers NE, Ortner M, et al. Link between hippocampus’ raised local and eased global intrinsic connectivity in AD. Alzheimers Dement. 2015;11(5):475–84. https://doi.org/10.1016/j.jalz.2014.02.007.
Irimia A, Van Horn JD. Systematic network lesioning reveals the core white matter scaffold of the human brain. Front Hum Neurosci. 2014;8:51. https://doi.org/10.3389/fnhum.2014.00051.
Bradshaw LA, Irimia A, Sims JA, Richards WO. Biomagnetic signatures of uncoupled gastric musculature. Neurogastroenterol Motil. 2009;21(7):778–e50. https://doi.org/10.1111/j.1365-2982.2009.01265.x.
Irimia A, Bradshaw LA. Artifact reduction in magnetogastrography using fast independent component analysis. Physiol Meas. 2005;26(6):1059–73. https://doi.org/10.1088/0967-3334/26/6/015.
Irimia A, Bradshaw LA. Ellipsoidal electrogastrographic forward modelling. Phys Med Biol. 2005;50(18):4429–44. https://doi.org/10.1088/0031-9155/50/18/012.
Irimia A, Richards WO, Bradshaw LA. Magnetogastrographic detection of gastric electrical response activity in humans. Phys Med Biol. 2006;51(5):1347–60. https://doi.org/10.1088/0031-9155/51/5/022.
Goh SYM, Irimia A, Vespa PM, Van Horn JD. Patient-tailored multimodal neuroimaging, visualization and quantification of human intra-cerebral hemorrhage. Proceedings of the SPIE Conference on Medical Imaging -- PACS and Imaging Informatics: Next Generation and Innovations 2016;9789.
Maher AS, Rostowsky KA, Chowdhury NF, Irimia A. Neuroinformatics and analysis of connectomic alterations due to cerebral microhemorrhages in geriatric mild neurotrauma. ACM BCB. 2018;2018:165–71. https://doi.org/10.1145/3233547.3233598.
Rostowsky KA, Maher AS, Irimia A. Macroscale white matter alterations due to traumatic cerebral microhemorrhages are revealed by diffusion tensor imaging. Front Neurol. 2018;9:948. https://doi.org/10.3389/fneur.2018.00948
Heringa SM, Reijmer YD, Leemans A, Koek HL, Kappelle LJ, Biessels GJ, et al. Multiple microbleeds are related to cerebral network disruptions in patients with early Alzheimer’s disease. J Alzheimers Dis. 2014;38(1):211–21. https://doi.org/10.3233/JAD-130542.
Irimia A, Van Horn JD. The structural, connectomic and network covariance of the human brain. Neuroimage. 2013;66:489–99. https://doi.org/10.1016/j.neuroimage.2012.10.066.
Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–42. https://doi.org/10.1073/pnas.0308627101.
Zhang HY, Wang SJ, Liu B, Ma ZL, Yang M, Zhang ZJ, et al. Resting brain connectivity: changes during the progress of Alzheimer disease. Radiology. 2010;256(2):598–606. https://doi.org/10.1148/radiol.10091701.
Hauck WW, Anderson S. A new statistical procedure for testing equivalence in two-group comparative bioavailability trials. J Pharmacokinet Biopharm. 1984;12(1):83–91. https://doi.org/10.1007/bf01063612.
Hilger K, Fukushima M, Sporns O, Fiebach CJ. Temporal stability of functional brain modules associated with human intelligence. Hum Brain Mapp. 2020;41(2):362–72. https://doi.org/10.1002/hbm.24807.
Hout MC, Papesh MH, Goldinger SD. Multidimensional scaling. Wiley Interdiscip Rev Cogn Sci. 2013;4(1):93–103. https://doi.org/10.1002/wcs.1203.
Irimia A, Goh SY, Torgerson CM, Stein NR, Chambers MC, Vespa PM, et al. Electroencephalographic inverse localization of brain activity in acute traumatic brain injury as a guide to surgery, monitoring and treatment. Clin Neurol Neurosurg. 2013;115(10):2159–65. https://doi.org/10.1016/j.clineuro.2013.08.003.
Irimia A, Goh SY, Torgerson CM, Chambers MC, Kikinis R, Van Horn JD. Forward and inverse electroencephalographic modeling in health and in acute traumatic brain injury. Clin Neurophysiol. 2013;124(11):2129–45.
Irimia A, Van Horn JD. Epileptogenic focus localization in treatment-resistant post-traumatic epilepsy. J Clin Neurosci. 2015;22(4):627–31.
Bronstein AM, Bronstein MM, Kimmel R. Generalized multidimensional scaling: a framework for isometry-invariant partial surface matching. Proc Natl Acad Sci U S A. 2006;103(5):1168–72. https://doi.org/10.1073/pnas.0508601103.
Gibson DB. Effect size as the essential statistic in developing methods for mTBI diagnosis. Front Neurol. 2015;6. https://doi.org/10.3389/fneur.2015.00126.
de Guise E, Alturki AY, LeBlanc J, Champoux MC, Couturier C, Lamoureux J, et al. The Montreal cognitive assessment in persons with traumatic brain injury. Appl Neuropsychol Adult. 2014;21(2):128–35. https://doi.org/10.1080/09084282.2013.778260.
Calvillo M, Irimia A. Neuroimaging and psychometric assessment of mild cognitive impairment after traumatic brain injury. Front Psychol. 2020;11:1423.
Lima EA, Irimia A, Wikswo JP. The magnetic inverse problem. In: Braginski JCA, editor. The SQUID Handbook: Applications of SQUIDs and SQUID Systems: Wiley-VCH; 2006.
Lima EA, Irimia A, Wikswo JP. The magnetic inverse problem. 2008. The SQUID Handbook, vol 2: Applications of SQUIDs and SQUID Systems. Clarke J., Braginski AI (Eds.). Wiley-VCH, pages 139–267.
Acknowledgments
The authors thank Maria Calvillo, Lei Cao, Yu Hu, Jun H. Kim, Sean O. Mahoney, Van Ngo, Kenneth A. Rostowsky, and Shania Wang for their assistance.
Computer code availability
The computer code used in this study is freely available. FreeSurfer and FS-FAST are freely available (https://surfer.nmr.mgh.harvard.edu). Equivalence testing was implemented using freely available MATLAB software (https://www.mathworks.com/matlabcentral/fileexchange/63204). Network analysis was implemented using the freely available Brain Connectivity Toolbox (https://sites.google.com/site/bctnet/). Network visualizations were generated using Gephi (http://gephi.org). Regression and SVM analyses were implemented in MATLAB (http://mathworks.com) using the glmfit, fitcsvm, and predict functions.
Funding
This work was supported by NIH grant R01 NS 100973 to A.I., by DoD award W81-XWH-1810413 to A.I., by a Hanson-Thorell Research Scholarship to A.I., and by the Undergraduate Research Associate Program (URAP) at the University of Southern California. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI, NIH Grant U01 AG024904) and DoD ADNI (DoD award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author information
Authors and Affiliations
Consortia
Contributions
A.I. contributed to the study design, data analysis, and result interpretation and wrote the manuscript. A.S.M., N.N.C., N.F.C., and E.B.J. contributed to the study design, data analysis, and result interpretation.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no actual or perceived competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
About this article
Cite this article
Irimia, A., Maher, A.S., Chaudhari, N.N. et al. Acute cognitive deficits after traumatic brain injury predict Alzheimer’s disease-like degradation of the human default mode network. GeroScience 42, 1411–1429 (2020). https://doi.org/10.1007/s11357-020-00245-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00245-6