Abstract
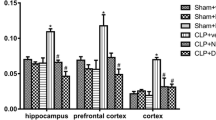
Sepsis causes organ dysfunction due to an infection, and it may impact the central nervous system. Neuroinflammation and oxidative stress are related to brain dysfunction after sepsis. Both processes affect microglia activation, neurotrophin production, and long-term cognition. Fish oil (FO) is an anti-inflammatory compound, and lipoic acid (LA) is a universal antioxidant substance. They exert neuroprotective roles when administered alone. We aimed at determining the effect of FO+LA combination on microglia activation and brain dysfunction after sepsis. Microglia cells from neonatal pups were co-treated with lipopolysaccharide (LPS) and FO or LA, alone or combined, for 24 h. Cytokine levels were measured. Wistar rats were subjected to sepsis by cecal ligation and perforation (CLP) and treated orally with FO, LA, or FO+LA. At 24 h after surgery, the hippocampus, prefrontal cortex, and total cortex were obtained and assayed for levels of cytokines, myeloperoxidase (MPO) activity, protein carbonyls, superoxide dismutase (SOD), and catalase (CAT) activity. At 10 days after surgery, brain-derived neurotrophic factor (BDNF) levels were determined and behavioral tests were performed. The combination diminished in vitro levels of pro-inflammatory cytokines. The combination reduced TNF-α in the cortex, IL-1β in the prefrontal cortex, as well as MPO activity, and decreased protein carbonyls formation in all structures. The combination enhanced catalase activity in the prefrontal cortex and hippocampus, elevated BDNF levels in all structures, and prevented behavioral impairment. In summary, the combination was effective in preventing cognitive damage by reducing neuroinflammation and oxidative stress and increasing BDNF levels.









Similar content being viewed by others
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR et al (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). J Am Med Assoc 315:801–810
Rello J, Valenzuela-Sánchez F, Ruiz-Rodriguez M, Moyano S (2017) Sepsis: A review of advances in management. Adv Ther 34:2393–2411. https://doi.org/10.1007/s12325-017-0622-8
Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, Kadri SS, Angus DC et al (2017) Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 318:1241–1249. https://doi.org/10.1001/jama.2017.13836
Lelubre C, Vincent JL (2018) Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol 14:417–427. https://doi.org/10.1038/s41581-018-0005-7
Adam N, Kandelman S, Mantz J, Chrétien F, Sharshar T (2013) Sepsis-induced brain dysfunction. Expert Rev Anti-Infect Ther 11:211–221. https://doi.org/10.1586/eri.12.159
Chaudhry N, Duggal AK (2014) Sepsis associated encephalopathy. Adv Med 2014:1–16. https://doi.org/10.1155/2014/762320
Annane D, Sharshar T (2014) Cognitive decline after sepsis. Lancet Respir Med 3:61–69. https://doi.org/10.1016/S2213-2600(14)70246-2
Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348:138–150. https://doi.org/10.1056/NEJMra021333
Victor VM, Espulgues JV, Hernandez-Mijares A, Rocha M (2009) Oxidative stress and mitochondrial dysfunction in sepsis: A potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets 9:376–389
Prauchner CA (2017) Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns 43:471–485. https://doi.org/10.1016/j.burns.2016.09.023
Gotts JE, Matthay MA (2016) Sepsis: Pathophysiology and clinical management. BMJ 353:1–20. https://doi.org/10.1136/bmj.i1585
Taeb AM, Hooper MH, Marik PE (2017) Sepsis: Current definition, pathophysiology, diagnosis, and management. Nutr Clin Pract 32:296–308. https://doi.org/10.1177/0884533617695243
Faix JD (2013) Biomarkers of sepsis. Crit Rev Clin Lab Sci 50:23–36. https://doi.org/10.3109/10408363.2013.764490
Michels M, Danielski LG, Dal-Pizzol F, Petronilho F (2014) Neuroinflammation: microglial activation during sepsis. Curr Neurovasc Res 11:2014–2015
Michels M, Vieira AS, Vuolo F, Zapelini HG, Mendonça B, Mina F, Dominguini D, Steckert A et al (2015) The role of microglia activation in the development of sepsis-induced long-term cognitive impairment. Brain Behav Immun 43:54–59. https://doi.org/10.1016/j.bbi.2014.07.002
Barichello T, Generoso JS, Simões LR, Steckert AV, Moreira AP, Dominguini D, Ferrari P, Gubert C et al (2015) Folic acid prevented cognitive impairment in experimental pneumococcal meningitis. J Neural Transm 122:643–651. https://doi.org/10.1007/s00702-014-1302-3
Allen SJ, Watson JJ, Shoemark DK, Barua NU, Patel NK (2013) GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol Ther 138:155–175. https://doi.org/10.1016/j.pharmthera.2013.01.004
Barichello T, Martins MR, Reinke A, Constantino LS, Machado RA, Valvassori SS, Moreira JCF, Quevedo J et al (2007) Behavioral deficits in sepsis-surviving rats induced by cecal ligation and perforation. Braz J Med Biol Res 40:831–837
Tuon L, Comim CM, Petronilho F, Barichello T, Izquierdo I, Quevedo J, Dal-Pizzol F (2008) Time-dependent behavioral recovery after sepsis in rats. Intensive Care Med 34:1724–1731. https://doi.org/10.1007/s00134-008-1129-1
Della Giustina A, Goldim MP, Danielski LG, Florentino D, Garbossa L, Joaquim L, Oliveira Junior AN, Mathias K et al (2019) Fish oil–rich lipid emulsion modulates neuroinflammation and prevents long-term cognitive dysfunction after sepsis. Nutrition 70:110417. https://doi.org/10.1016/j.nut.2018.12.003
Zarbato GF, de Souza Goldim MP, Della GA et al (2018) Dimethyl fumarate limits neuroinflammation and oxidative stress and improves cognitive impairment after polymicrobial sepsis. Neurotox Res 34:418–430. https://doi.org/10.1007/s12640-018-9900-8
Della Giustina A, Goldim MP, Danielski LG, Florentino D, Mathias K, Garbossa L, Oliveira Junior AN, Fileti ME et al (2017) Alpha-lipoic acid attenuates acute neuroinflammation and long-term cognitive impairment after polymicrobial sepsis. Neurochem Int 108:436–447. https://doi.org/10.1016/j.neuint.2017.06.003
Barichello T, Machado RA, Constantino L, Valvassori SS, Réus GZ, Martins MR, Petronilho F, Ritter C et al (2007) Antioxidant treatment prevented late memory impairment in an animal model of sepsis. Crit Care Med 35:2186–2190. https://doi.org/10.1097/01.CCM.0000281452.60683.96
Calder PC (2012) The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res 56:1073–1080. https://doi.org/10.1002/mnfr.201100710
Calder PC (2014) Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 1851:469–484. https://doi.org/10.1016/j.bbalip.2014.08.010
Li CC, Yang HT, Hou YC, Chiu YS, Chiu WC (2014) Dietary fish oil reduces systemic inflammation and ameliorates sepsis-induced liver injury by up-regulating the peroxisome proliferator-activated receptor gamma-mediated pathway in septic mice. J Nutr Biochem 25:19–25. https://doi.org/10.1016/j.jnutbio.2013.08.010
Seifar F, Khalili M, Khaledyan H, Amiri Moghadam S, Izadi A, Azimi A, Shakouri SK (2017) α-Lipoic acid, functional fatty acid, as a novel therapeutic alternative for central nervous system diseases: A review. Nutr Neurosci 22:1–11. https://doi.org/10.1080/1028415X.2017.1386755
Hiller S, DeKroon R, Hamlett ED, Xu L, Osorio C, Robinette J, Winnik W, Simington S et al (2016) Alpha-lipoic acid supplementation protects enzymes from damage by nitrosative and oxidative stress. Biochim Biophys Acta Gen Subj 1860:36–45. https://doi.org/10.1016/j.bbagen.2015.09.001
Takechi R, Pallebage-Gamarallage MM, Lam V, Giles C, Mamo JC (2013) Nutraceutical agents with anti-inflammatory properties prevent dietary saturated-fat induced disturbances in blood-brain barrier function in wild-type mice. J Neuroinflammation 10:73. https://doi.org/10.1186/1742-2094-10-73
Ahmadi A, Mazooji N (2013) Effect of alpha-lipoic acid and vitamin E supplementation on oxidative stress, inflammation, and malnutrition in hemodialysis patients. Iran J Kidney Dis 7:461–467
Al-Rasheed NM, Al-Rasheed NM, Attia HA et al (2013) Adverse cardiac responses to alpha-lipoic acid in a rat-diabetic model: Possible mechanisms? J Physiol Biochem 69:761–778. https://doi.org/10.1007/s13105-013-0252-9
Castro MC, Francini F, Gagliardino JJ, Massa ML (2014) Lipoic acid prevents fructose-induced changes in liver carbohydrate metabolism: Role of oxidative stress. Biochim Biophys Acta 1840:1145–1151. https://doi.org/10.1016/j.bbagen.2013.12.005
Tamashiro TT, Dalgard CL, Byrnes KR (2012) Primary microglia isolation from mixed glial cell cultures of neonatal rat brain tissue. J Vis Exp:1–5. https://doi.org/10.3791/3814
Bronstein R, Torres L, Nissen JC, Tsirka SE (2013) Culturing microglia from the neonatal and adult central nervous system. J Vis Exp:1–6. https://doi.org/10.3791/50647
Lian H, Roy E, Zheng H (2016) Protocol for primary microglial culture preparation. Bio Protoc 6:1–10. https://doi.org/10.21769/BioProtoc.1989
Park SY, Jin ML, Ko MJ, Park G, Choi YW (2016) Anti-neuroinflammatory effect of emodin in LPS-stimulated microglia: Involvement of AMPK/Nrf2 activation. Neurochem Res 41:2981–2992. https://doi.org/10.1007/s11064-016-2018-6
Scumpia PO, Kelly-Scumpia K, Stevens BR (2014) Alpha-lipoic acid effects on brain glial functions accompanying double-stranded RNA antiviral and inflammatory signaling. Neurochem Int 64:55–63. https://doi.org/10.1016/j.neuint.2013.11.006
Razzak A, Aldrich C, Babcock TA, Saied A, Espat NJ (2008) Attenuation of iNOS in an LPS-stimulated macrophage model by omega-3 fatty acids is independent of COX-2 derived PGE2. J Surg Res 145:244–250. https://doi.org/10.1016/j.jss.2007.07.003
Fink MP, Heard SO (1990) Laboratory models of sepsis and septic shock. J Surg Res 49:186–196
Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4:31–36. https://doi.org/10.1038/nprot.2008.214
Alwayn IPJ, Gura K, Nosé V, Zausche B, Javid P, Garza J, Verbesey J, Voss S et al (2005) Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res 57:445–452. https://doi.org/10.1203/01.PDR.0000153672.43030.75
Petronilho F, Florentino D, Danielski LG, Vieira LC, Martins MM, Vieira A, Bonfante S, Goldim MP et al (2015) Alpha-lipoic acid attenuates oxidative damage in organs after sepsis. Inflammation 39:357–365. https://doi.org/10.1007/s10753-015-0256-4
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, second. Academic Press, Sydney
De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989) Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26:335–341
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
George A, Schmidt C, Weishaupt A, Toyka KV, Sommer C (1999) Serial determination of tumor necrosis factor-alpha content in rat sciatic nerve after chronic constriction injury. Exp Neurol 160:124–132. https://doi.org/10.1006/exnr.1999.7193
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carvalho D, Petronilho F, Vuolo F, Machado RA, Constantino L, Guerrini R, Calo G, Gavioli EC et al (2008) The nociceptin/orphanin FQ-NOP receptor antagonist effects on an animal model of sepsis. Intensive Care Med 34:2284–2290. https://doi.org/10.1007/s00134-008-1313-3
Iacobone E, Bailly-Salin J, Polito A, Friedman D, Stevens RD, Sharshar T (2009) Sepsis-associated encephalopathy and its differential diagnosis. Crit Care Med 37:S331–S336. https://doi.org/10.1097/CCM.0b013e3181b6ed58
Bozza FA, D’Avila JC, Ritter C, Sonneville R, Sharshar T, Dal-Pizzol F (2013) Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock 39:10–16. https://doi.org/10.1097/SHK.0b013e31828fade1
Sharshar T, Bozza F, Chrétien F (2014) Neuropathological processes in sepsis. Lancet Neurol 13:534–536. https://doi.org/10.1016/S1474-4422(14)70064-X
Kim Y-K, Na K-S, Myint A-M, Leonard BE (2016) The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 64:277–284. https://doi.org/10.1016/J.PNPBP.2015.06.008
Gray SM, Bloch MH (2012) Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr Psychiatry Rep 14:220–228. https://doi.org/10.1007/s11920-012-0272-0
Chaudhry H, Zhou J, Zhong Y et al (2013) Role of cytokines as a double-edged sord in sepsis. In Vivo 27:669–684
Plaschke K, Fichtenkamm P, Schramm C, Hauth S, Martin E, Verch M, Karck M, Kopitz J (2010) Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med 36:2081–2089. https://doi.org/10.1007/s00134-010-2004-4
Katsumata Y, Harigai M, Kawaguchi Y, Fukasawa C, Soejima M, Takagi K, Tanaka M, Ichida H et al (2007) Diagnostic reliability of cerebral spinal fluid tests for acute confusional state (delirium) in patients with systemic lupus erythematosus: Interleukin 6 (IL-6), IL-8, interferon-alpha, IgG index, and Q-albumin. J Rheumatol 34:2010–2017
Sun J, Zhang S, Zhang X, Zhang X, Dong H, Qian Y (2015) IL-17A is implicated in lipopolysaccharide-induced neuroinflammation and cognitive impairment in aged rats via microglial activation. J Neuroinflammation 12:165. https://doi.org/10.1186/s12974-015-0394-5
Perry VH (2004) The influence of systemic inflammation on inflammation in the brain: Implications for chronic neurodegenerative disease. Brain Behav Immun 18:407–413. https://doi.org/10.1016/j.bbi.2004.01.004
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462. https://doi.org/10.1002/glia.20467
Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, Chretien F, Sharshar T (2013) Understanding brain dysfunction in sepsis. Ann Intensive Care 3:1–11. https://doi.org/10.1186/2110-5820-3-15
Michels M, Danieslki LG, Vieira A, Florentino D, Dall’Igna D, Galant L, Sonai B, Vuolo F et al (2015) CD40–CD40 ligand pathway is a major component of acute neuroinflammation and contributes to long-term cognitive dysfunction after sepsis. Mol Med 21:219–226. https://doi.org/10.2119/molmed.2015.00070
Danielski LG, Della GA, Badawy M et al (2017) Brain barrier breakdown as a cause and consequence of neuroinflammation in sepsis. Mol Neurobiol 55:1–9. https://doi.org/10.1007/s12035-016-0356-7
Hoogland ICM, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D (2015) Systemic inflammation and microglial activation: Systematic review of animal experiments. J Neuroinflammation 12:114. https://doi.org/10.1186/s12974-015-0332-6
Guo Q, Tirosh O, Packer L (2001) Inhibitory effect of alpha-lipoic acid and its positively charged amide analogue on nitric oxide production in RAW 264.7 macrophages. Biochem Pharmacol 61:547–554. https://doi.org/10.1016/s0006-2952(00)00569-4
Demarco VG, Scumpia PO, Bosanquet JP, Skimming JW (2004) Alpha-lipoic acid inhibits endotoxin-stimulated expression of iNOS and nitric oxide independent of the heat shock response in RAW 264.7 cells. Free Radic Res 38:675–682
Shen H-H, Lam K-K, Cheng P-Y, Kung CW, Chen SY, Lin PC, Chung MT, Lee YM (2015) Alpha-lipoic acid prevents endotoxic shock and multiple organ dysfunction syndrome induced by endotoxemia in rats. Shock 43:405–411. https://doi.org/10.1097/SHK.0000000000000295
Koriyama Y, Nakayama Y, Matsugo S, Sugitani K, Ogai K, Takadera T, Kato S (2013) Anti-inflammatory effects of lipoic acid through inhibition of GSK-3B in lipopolysaccharide-induced BV-2 microglial cells. Neurosci Res 77:87–96. https://doi.org/10.1016/j.neures.2013.07.001
Monk JM, Liddle DM, De Boer AA et al (2015) Fish-oil–derived n–3 PUFAs reduce inflammatory and chemotactic adipokine-mediated cross-talk between co-cultured murine splenic CD8+ T cells and adipocytes. J Nutr 145:829–838. https://doi.org/10.3945/jn.114.205443
De Boer AA, Monk JM, Liddle DM et al (2016) Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross-talk between adipocytes and CD11b+ macrophages. J Nutr Biochem 34:61–72. https://doi.org/10.1016/J.JNUTBIO.2016.04.004
Cotogni P, Trombetta A, Muzio G, Maggiora M, Canuto RA (2015) The Omega-3 fatty acid docosahexaenoic acid modulates inflammatory mediator release in human alveolar cells exposed to Bronchoalveolar lavage fluid of ARDS patients. Biomed Res Int 2015:1–11. https://doi.org/10.1155/2015/642520
Wijendran V, Brenna JT, Wang DH, Zhu W, Meng D, Ganguli K, Kothapalli KSD, Requena P et al (2015) Long-chain polyunsaturated fatty acids attenuate the IL-1β-induced proinflammatory response in human fetal intestinal epithelial cells. Pediatr Res 78:626–633. https://doi.org/10.1038/pr.2015.154
Reddy RC, Standiford TJ (2010) Effects of sepsis on neutrophil chemotaxis. Curr Opin Hematol 17:18–24. https://doi.org/10.1097/MOH.0b013e32833338f3
Kovach MA, Standiford TJ (2012) The function of neutrophils in sepsis. Curr Opin Infect Dis 25:321–327. https://doi.org/10.1097/QCO.0b013e3283528c9b
Amanzada A, Malik IA, Nischwitz M, Sultan S, Naz N, Ramadori G (2011) Myeloperoxidase and elastase are only expressed by neutrophils in normal and in inflammed liver. Histochem Cell Biol 135:305–315. https://doi.org/10.1007/s00418-011-0787-1
Costantini C, Cassatella MA (2011) The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J Leukoc Biol 89:221–233. https://doi.org/10.1189/jlb.0510250
He H, Geng T, Chen P, Wang M, Hu J, Kang L, Song W, Tang H (2016) NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Sci Rep 6:27711. https://doi.org/10.1038/srep27711
Andonegui G, Zelinski EL, Schubert CL, Knight D, Craig LA, Winston BW, Spanswick SC, Petri B et al (2018) Targeting inflammatory monocytes in sepsis-associated encephalopathy and long-term cognitive impairment. JCI Insight 3:1–20. https://doi.org/10.1172/JCI.INSIGHT.99364
Danielski LG, Della GA, Goldim MP et al (2017) Vitamin B6 reduces neurochemical and long-term cognitive alterations after polymicrobial sepsis: Involvement of the kynurenine pathway modulation. Mol Neurobiol 55:1–14. https://doi.org/10.1007/s12035-017-0706-0
Vieira AA, Michels M, Florentino D, Nascimento D, Rezin G, Leffa D, Fortunato J, Dal-Pizzol F et al (2015) Obesity promotes oxidative stress and exacerbates sepsis-induced brain damage. Curr Neurovasc Res 12:147–154
Fialkow L, Wang Y, Downey GP (2007) Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med 42:153–164. https://doi.org/10.1016/j.freeradbiomed.2006.09.030
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322. https://doi.org/10.1104/pp.106.077073
Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A (2003) Protein carbonylation in human diseases. Trends Mol Med 9:169–176. https://doi.org/10.1016/S1471-4914(03)00031-5
Denny Joseph KM, Muralidhara M (2014) Neuroprotective efficacy of a combination of fish oil and ferulic acid against 3-nitropropionic acid-induced oxidative stress and neurotoxicity in rats: Behavioural and biochemical evidence. Appl Physiol Nutr Metab 39:487–496. https://doi.org/10.1139/apnm-2013-0262
Denny Joseph KM, Muralidhara M (2013) Enhanced neuroprotective effect of fish oil in combination with quercetin against 3-nitropropionic acid induced oxidative stress in rat brain. Prog Neuro-Psychopharmacol Biol Psychiatry 40:83–92. https://doi.org/10.1016/J.PNPBP.2012.08.018
Ritter C, Andrades M, Frota MLC et al (2003) Oxidative parameters and mortality in sepsis induced by cecal ligation and perforation. Intensive Care Med 29:1782–1789. https://doi.org/10.1007/s00134-003-1789-9
Ritter C, Andrades ME, Reinke A, Menna-Barreto S, Moreira JCF, Dal-Pizzol F (2004) Treatment with N-acetylcysteine plus deferoxamine protects rats against oxidative stress and improves survival in sepsis. Crit Care Med 32:342–349. https://doi.org/10.1097/01.CCM.0000109454.13145.CA
Barichello T, Generoso JS, Simões LR, Faller CJ, Ceretta RA, Petronilho F, Lopes-Borges J, Valvassori SS et al (2014) Sodium butyrate prevents memory impairment by re-establishing BDNF and GDNF expression in experimental pneumococcal meningitis. Mol Neurobiol 52:734–740. https://doi.org/10.1007/s12035-014-8914-3
Denny Joseph KM, Muralidhara M (2015) Combined oral supplementation of fish oil and quercetin enhances neuroprotection in a chronic rotenone rat model: Relevance to Parkinson’s disease. Neurochem Res 40:894–905. https://doi.org/10.1007/s11064-015-1542-0
Li G, Gao L, Jia J, Gong X, Zang B, Chen W (2014) alpha-Lipoic acid prolongs survival and attenuates acute kidney injury in a rat model of sepsis. Clin Exp Pharmacol Physiol 41:459–468. https://doi.org/10.1111/1440-1681.12244
Körner A, Schlegel M, Theurer J, Frohnmeyer H, Adolph M, Heijink M, Giera M, Rosenberger P et al (2018) Resolution of inflammation and sepsis survival are improved by dietary Ω-3 fatty acids. Cell Death Differ 25:421–431. https://doi.org/10.1038/cdd.2017.177
Park Y, Nam S, Yi H-J, Hong HJ, Lee M (2009) Dietary n-3 polyunsaturated fatty acids increase oxidative stress in rats with intracerebral hemorrhagic stroke. Nutr Res 29:812–818. https://doi.org/10.1016/J.NUTRES.2009.10.019
Tsuduki T, Honma T, Nakagawa K, Ikeda I, Miyazawa T (2011) Long-term intake of fish oil increases oxidative stress and decreases lifespan in senescence-accelerated mice. Nutrition 27:334–337. https://doi.org/10.1016/J.NUT.2010.05.017
Ide T (2018) Physiological activities of the combination of fish oil and α-lipoic acid affecting hepatic lipogenesis and parameters related to oxidative stress in rats. Eur J Nutr 57:1545–1561. https://doi.org/10.1007/s00394-017-1440-0
Funding
This research was supported by grants from the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq) (FP) and the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES) (ADG). FP and TB are CNPq Research Fellows. The funding sources were not involved in the conduction of the research, preparation of the article nor in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
FB and ADG conceived, designed, and coordinated the in vivo study. TB and ADG conceived, designed, and coordinated the in vitro study. ADG and VVG prepared microglia cell culture. ADG, GS, and GC performed microglia cell treatment and cytokine analysis. ADG, MPSG, LGD, and AON performed sepsis induction and treatments. AON, LG, and TC ensured and provided animal care throughout the in vivo study. JP, BHO, DFM, and FB performed neurotrophin determinations. MG, TB-S, and JB performed cytokine measurements. ADG, LG, TD, TC, and SB performed oxidative stress and myeloperoxidase determinations. NR and JJF performed behavioral evaluations. ADG analyzed the data, prepared the figures, and wrote the manuscript. FP, TB, and ADG revised and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Animal Welfare Committee Animal Research of University of Texas Health Science Center at Houston – United States, protocol number AWC-15-0056; Animal Research Ethic Committee of the Universidade do Sul de Santa Catarina - Brazil, protocol number 15.043.4.01.IV). All efforts were made to minimize the number of animals used and their suffering.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giustina, A.D., de Souza Goldim, M.P., Danielski, L.G. et al. Lipoic Acid and Fish Oil Combination Potentiates Neuroinflammation and Oxidative Stress Regulation and Prevents Cognitive Decline of Rats After Sepsis. Mol Neurobiol 57, 4451–4466 (2020). https://doi.org/10.1007/s12035-020-02032-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02032-y