Abstract
Antimicrobial resistance (AMR) is one of the significant clinical challenges and also an emerging area of concern arising from nosocomial infections of ESKAPE pathogens, which has been on the rise in both the developed and developing countries alike. These pathogens/superbugs can undergo rapid mutagenesis, which helps them to generate resistance against antimicrobials in addition to the patient’s non-adherence to the antibiotic regimen. Sticking to the idea of a ‘one-size-fits-all’ approach has led to the inappropriate administration of antibiotics resulting in augmentation of antimicrobial resistance. Antimicrobial peptides (AMPs) are the natural host defense peptides that have gained attention in the field of AMR, and recently, synthetic AMPs are well studied to overcome the drawbacks of natural counterparts. This review deals with the novel techniques utilizing the bacteriolytic activity of natural AMPs. The effective localization of these peptides onto the negatively charged bacterial surface by using nanocarriers and structurally nanoengineered antimicrobial peptide polymers (SNAPPs) owing to its smaller size and better antimicrobial activity is also described here.
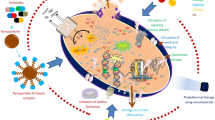
Graphic abstract

Similar content being viewed by others
Introduction
A variety of organisms, including bacteria, fungi, viruses, and parasites, are mainly responsible for causing nosocomial infections. The surveillance studies from the hospital and Infectious Diseases Society of America has designated the group of pathogens responsible for causing nosocomial infections as ESKAPE pathogens. ESKAPE pathogens are a group of bacteria containing both Gram-positive and Gram-negative bacteria, namely, Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. As per the WHO reports, the mortalities due to drug resistant-strains of ESKAPE bugs such as Klebsiella pneumoniae, Escherichia coli, Neisseria gonorrhoeae, and Staphylococcus aureus have increased in the past few years. Analysis of the bacterial genomes has led to the conclusion that there is a shortage of potent antibiotics because around 20,000 potential resistant genes are reported. The USA alone has showed 99,000 deaths, which were associated with the hospital-acquired infections. The two most common hospital-acquired diseases are sepsis, and pneumonia which has caused around 55,000 deaths resulting in both microbial as well as economic burden (Aslam et al. 2018; Dixit et al. 2019). ESKAPE bugs infections have been growing in both the developed and the developing countries alike (Rice 2008).
The first and foremost reason behind the spread of nosocomial infection is the poor hygienic conditions, sanitation, and malnourishment. But, the most challenging part of this epidemic is its treatment. Physicians prescribe an enormous number of combinations of antibiotics without considering its side effects. Even if a disease whose cause may be a narrow spectrum bacterium which can be treated by a single low dose antibiotic regimen, they are being forced to be processed by giving high dose combinations to overcome the antimicrobial resistance (AMR). The concept of antimicrobial resistance comes from the fact of inadequate antibacterial therapy, which deals with treating the bacterial disease with an antibiotic to which the bacteria are not susceptible. This leads to an increased resistance in the circulating bacterial agents in the human body. Inappropriate or overuse of antibiotics may also lead to antimicrobial resistance (Olesen et al. 2018). Among ESKAPE pathogens, methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum β-lactamase producing (ESBL) Escherichia coli and vancomycin-resistant Enterococcus (VRE) are commonly seen. Although these agents have gained popularity, they exert resistance against antimicrobial agents in the healthcare set-up. A relationship of resistance between the host-immune responses with the frequency of biofilm formation has already been established (Tenover 2006; Murali et al. 2014). WHO has come out with a global priority list of antibiotic-resistant bacteria which guides in the new antibiotic development (Table 1). Certain pathogens have been grouped as ESKAPE pathogens by the WHO against which new antibiotics are essential. The discovery of novel therapy for the treatment of drug-resistant infections, particularly those caused by ESKAPE pathogens is time-consuming. Hence, antibiotics in conjunctions, synthetic chemicals, phages, antimicrobial peptides (AMPs), nanomaterials, and photodynamic light therapy have been recommended as an alternative method (Mulani et al. 2019; Ma et al. 2020). Out of these measures, AMPs, natural host defense peptides, containing a backbone of amino acids are found to be promising candidates, which can be an alternative to the conventional antibiotics that develop resistance. Although AMPs have shown successful results,they also suffer from various drawbacks such as toxicity, susceptibility to proteolysis, poor pharmacokinetics profile, and many more. The development of nanocarriers or nanomaterials encapsulated with AMPs helps in minimizing the degradation and cytotoxicity with increased efficiency of AMPs at the target site (Brandelli 2012). In addition to nanocarriers, the star polymers and structurally nanoengineered antimicrobial peptide polymers are also used to deliver the AMPs due to its significant advantages and potential therapeutic efficacy (Lam et al. 2016).
This review deals with the AMPs with its mechanism of action and bacteriolytic activity against combating the ESKAPE crisis. Further, the emphasis is given on the delivery of AMPs using nanocarriers, novel nanostructures such as star polymers, and structurally nanoengineered antimicrobial peptide polymers (SNAPPs). The effective localization of these peptides onto the negatively charged bacterial surface owing to its smaller size which may enhance the antimicrobial activity is also discussed.
Antimicrobial peptides (AMPs)
AMPs are the peptides produced naturally by the multicellular organisms as the first line of defense against pathogenic microbes during infections. AMPs are amphiphilic in nature with cationic charge and relatively smaller in size (10–50 amino acids). Human have an innate immunity to microbial infections such as lysozyme secreted by the nasal mucosa which acts as a bacteriolytic. These enzymes are polypeptide in nature. Polypeptides are widely being known for their characteristic size that might exist in different conformation such as primary, secondary, tertiary, quaternary which adds to its flexibility, amphiphilic nature, and surface charge, which is complementary to the cell membrane surface charge of the bacteria. Subgroup I anionic peptides contain zinc (Zn) as a cofactor, which is essential for the antimicrobial efficacy, and subgroup II lacks cysteine residue,and forms a α-helical structures in the presence of sodium dodecyl sulfate (Hancock and Sahl 2006). There are different mechanisms by which it hampers or obstructs the resistance development. It targets the cell wall of bacteria by forming electrostatic interactions between the anionic bacterial cell wall membranes with AMPs cationic residues, which shows the bactericidal activity and, in addition to this, the insertion of hydrophilic subunits into the cytoplasmic membrane of bacteria leads to the permeabilization or disruption, thus causing the cell death. AMPs also act by forming the pores on the bacterial cell membrane which causes the death of the bacteria (Kamaruzzaman et al. 2019; Namivandi-Zangeneh et al. 2019). Since then, various classes of AMPs have been identified which are listed out in Table 2 (Ashley et al. 2018).
The characteristics of an ideal antimicrobial peptide are listed below: (Brogden 2005):
-
Size: approximately 6–59 amino acids chain length and anionic nature
-
Sequencing: Should contain basic amino acid residues like arginine/lysine, hydrophobic residues like alanine, leucine, and phenylalanine. The ratio of hydrophobic to a charged concentration should be 1:1 or 2:1.
-
Configuration: Preferably α-helical. Some of the AMPs are found in the form of two antiparallel β-sheets (γ-core motif).
-
Sufficiently hydrophobic to partition through the cell membrane.
-
Amphipathicity, expressed as a hydrophobic moment, summing up all the hydrophobic vector residues in the helical structure that are easy to calculate compared to those peptides with spatial configuration.
Mechanism of action of AMPs
Two basic mechanisms of short cationic amphiphilic host defense peptides responsible for the antimicrobial activity are direct cell killing and immunomodulatory action. Several models have been proposed to explain the disruption of the cell membrane caused by the AMPs. Amongst them, the 'Barrel-stave', 'Carpet model', and 'Toroidal-pore' are the major ones (Fig. 1) (Zasloff 2019). AMPs mediated cell killing involves three basic steps namely, attraction, attachment, and peptide insertion. Attraction step involves the basic electrostatic bonding of the charged cationic/anionic peptides and negatively charged units of the bacterial surface. In the attachment step, the peptide must penetrate the whole distance of the polysaccharide bacterial surface and join with the lipopolysaccharide, especially from Gram-negative bacteria or teichoic and lipoteichoic acid from Gram-positive bacteria. The α-helical structures are more effective as they can attach the bacterial membrane at even low peptide/lipid ratios. Following the attachment, any of the above said models are applied for creating a pore in the cell membrane surface, thus disrupting the cell membrane integrity. At the initial stages, I-state in which the peptides are parallel to the lipid surface are formed. As the concentration of peptide increases, it aligns itself perpendicular to the cell membrane. In the 'Barrel-stave' model, the hydrophobic region aligns itself towards the lipidic portion, and the hydrophilic region forms the inside portion of the pore (Fig. 2a). In the 'Carpet model,' the peptides are electrostatically bonded to the negatively charged cell membrane in such a way that it is spread all over (Fig. 2b). Antimicrobial activity is exerted upon the disruption of the cell membrane at higher peptide concentrations. In the 'Toroidal pore' model, the inserted peptides cause bending of the lipidic portions in such a way which gives a structure of pore (Fig. 2c) (Dar et al. 2016).
General mechanism of action of antimicrobial peptides; a represents attraction step where electrostatic bonding arises between negatively charged peptidoglycan layer of the bacterial cell membrane and amphiphilic polypeptide structure; b represents attachment step where the AMPs binds to Lipopolysaccharide (LPS) layer of Gram-negative cell wall and a teichoic acid layer of Gram-positive cell wall; c Represents the final peptide insertion step where following attachment the peptide forms a pore and thus disrupts the bacterial cell membrane
a Barrel-stave model of pore formation by AMPs, the hydrophobic region marked by red color aligns itself to the lipophilic part of the phospholipid bilayer, and the hydrophilic part represented by small orange part aligns itself towards the hydrophilic region of the phospholipid bilayer. b Carpet model of AMPs induced cell killing. Electrostatic bonding between the negatively charged bacterial cell surface and polypeptides aligns parallel to the cell membrane. c Toroidal pore model. AMPs induce the bending of the lipid monolayer in such a way that the polar head groups are both associated with the inserted peptides as well as the lipophilic
Apart from the physical disruption as pore formers, AMPs also exert their intracellular killing activity by the metabolic modulators. Activating apoptotic behavior of the bacterial cell by upregulation of autolysins e.g. N-acetylmuramoyl-L-alanine which acts as an autolysin activator, modulators of DNA replication like Buforin II; inhibition of DNA, RNA and protein synthesis by pleurocidin, dermaseptin, HNP-1, HNP-2 and inhibition of enzymatic activity by histatins, drosocin, apidaecin have been the proposed mechanisms of these peptides.
Peptide-based antimicrobial products
AMPs are made either by non-ribosomal peptide synthesis or ribosomal translation of mRNA. Recently, the ribosomal derived peptides produced by all species, have gained wide therapeutic potential (Mahlapuu et al. 2016). Conventional techniques of solid-phase peptide synthesis methods have not been up to the mark in peptide-based antibiotic development technology. Increasing the spectrum of activity and cost-effectiveness are the two main factors that have been focused upon to achieve a reliable formulation objective. Preparation of the peptide arrays involves the incorporation of smaller peptide fragments on the spot using cellulose sheets and then determining its antimicrobial efficacy. Development of peptidomimetic compounds that contains analogs which block the synthesis of a certain enzyme, can be used to inhibit protease synthesis, which resolves the stability issue. (Dar et al. 2016).
Synthetic approach to synthesize such antimicrobial peptides, however, has led to an excellent development in the field of peptide-based antimicrobials. Nevertheless, even notorious microorganisms like P. aeruginosa and E. coli have shown the tendency to acquire resistance against such products due to their ability of rapid mutagenesis (Mendelson et al. 2016, 2017). The high osmolarity, flavonoids, phytochemical constituents (methyl glyoxal which is 1,2 dicarbonyl compound), lysine/ arginine side chains are involved in the uptake of these constituents in certain diseases. These components of manuka honey and its importance in combating the antimicrobial resistance of P. aeruginosa have been described by Shenoy et al. and Alvarez-Suarez et al. (Shenoy et al. 2012; Alvarez-Suarez et al. 2014).
Resistance to AMPs
Similar to the conventional and other modified dosage forms of antibiotics, bacteria have shown resistance against these AMPs. The mechanism by which resistance is induced includes the alteration of the bacterial cell surface to release the various proteolytic enzymes which results in the hydrolysis of the peptides, for e.g. S. aureus alters the net surface charge towards a less negative by introducing basic groups like D-ala, and K. pneumoniae forms a capsular body which limits the penetration of the AMPs. Increasing the fluidity of the outer membrane surface by alteration of the lipid A portion as in Salmonella spp., modulation of the outer membrane proteins as in Yersinia Kaczmarek enterocolitica (Hay et al. 2018), presence of active efflux transporters and presence of proteolytic enzymes like metalloproteinase (aureolysin) in S. aureus has resulted in an increased resistance to AMPs. For specific Gram-negative bacteria, especially Enterobacteriaceae, they exert resistant mechanisms in the form of adaptation to AMPs, proteolytic degradation of AMPs, and shielding of the bacterial cell surface, thus limiting the penetration of AMPs. PhoPQ, PmrAB, and RcsBCD Phosphorelay system are the signaling pathways controlled by the genes of Enterobacteriaceae, which codes for AMPs resistance. Proteolytic enzymes like elastase from P. aeruginosa have been found to inactivate AMPs LL-37. Most of the periodontal disease-causing microbes like Porphyromonas gingivalis secrete AMPs thus inactivating the enzymes like proteases. Proteases released by the outer membrane site is the primary cause of AMPs degradation in Enterobacteriaceae spp. Formation of capsule polysaccharides as in K. pneumoniae, complex formation of AMPs with exopolysaccharides as in P. aeruginosa, and modification of the O-polysaccharide in the outer membrane site (Gruenheid and Le Moual 2012) are the major steps involved in shielding of the bacterial cell surface against AMPs.
The emergence of resistance to conventional antibiotics by several microorganisms has further augmented the research in the case of AMPs. AMPs have played an important role as a self-defense mechanism as well as penetration enhancer for certain antibiotics (Chowdhury et al. 2018; Fontela et al. 2018). Many novel drug delivery systems were attempted to deliver AMPs and to reduce their resistance. Table 3 provides information about the latest antimicrobial peptides, which are currently under clinical trials (https://clinicaltrials.gov/, NIH).
Novel delivery approaches for AMPs
There is a higher occurrence of bacterial infection with the development of the bacterial resistance to the conventional antibiotics, which was thought to be solved by the generation of new antibiotics or the development of AMPs. AMPs are of natural origin, which is effective in combating antimicrobial resistance in the place of conventional antibiotics. Nonetheless, development of these newer antibiotics (AMPs) restricts its use due to various problems such as undesirable or nonspecific interactions, proteolytic degradation, and cytotoxicity with limited in vivo activity as well as the stability and selectivity which make the AMPs inefficient to reach the target and exert its action.
Researchers have attempted to develop novel formulation systems for delivering the AMPs, which may help in avoiding the problems associated with delivering AMPs alone. Thus, encapsulating the AMPs into different nanocarriers may provide the direct application for targeting the AMPs in alternate ways. Various scientists have worked on different nanocarriers and successfully encapsulated the AMPs in it and targeted for the AMR therapy such as novel polymeric and lipidic nanoparticles, carbon nanotubes, micelles, liposomes and cubosomes, polymersomes, microspheres, dendrimers, nanocapsules, and other colloidal delivery systems (size up to a few hundred nanometers). These nanocarriers play the role of the transporters to deliver the encapsulated AMPs into the cells which are infected or to the intracellular pathogens. The development of nano formulations loaded with AMPs can aid in avoiding poor bioavailability, proteolysis, or susceptibility and toxicity associated with APMs. Additionally, conjugation of the AMPs with functional polymer provides an excellent antimicrobial activity with new functionalities and also reduces the toxicity by improving its selectivity (Sun et al. 2018). Thus, the development of novel nanocarriers and polymer conjugation concept opens new avenues for the translation of AMPs and its formulations from bench to bedside, However, only a few of the AMPs and its formulations have been translated to clinical trials. The detailed description of different nanocarriers used for delivering the AMPs with their targets is given in Table 4. The formulation strategies explored for the antimicrobial peptides are shown in Fig. 3. (Brandelli 2012; Carmona-Ribeiro and Carrasco 2014; Chen et al. 2014; Almaaytah et al. 2017; Shao et al. 2019; Makowski et al. 2019).
Formulation strategies of Antimicrobial peptides (Martin-Serrano et al. 2019)
Researchers have also worked on other novel nanomaterials in addition to the nanocarriers mentioned in Table 4, which are less susceptible to developing resistance against the antibiotics. These novel nanomaterials are star peptide polymers and structurally nanoengineered antimicrobial peptide polymers (SNAPPs). The star-shaped polymers are useful in killing bacteria, which has been proven by in vitro and in vivo studies. In addition, they are less toxic, and it acts through multiple pathways, which may not be possible with conventional antibiotics, thus making this nanocarrier more popular than the conventional nanocarriers (Australasian Science Magazine 2016).
Star polymers: a ray of hope
The novel delivery system approach has evolved enormously using different polymeric structures to enhance the stability, biocompatibility, and therapeutic efficacy of the drugs. Targeting moieties, hydrophobic, and hydrophilic polymeric carriers, nanoparticles are some of the significant approaches undertaken to improve drug delivery. Star polymers are one such type of delivery system which has gained importance in the field of biomedical applications, starting from gene delivery to antibacterial therapy. Unlike dendrimers, star polymers form a simpler structure which consists of linear arms (unlike dendrimers which consist of branched arms). Certain characteristics like the introduction of functional groups, lower solution viscosity (simpler structure), and biocompatibility have gained an interest in the area of biomedical research (Table 5). The multifunctional core with at least three macromolecular chains representing a star-shaped polymeric structure can attach to a targeting moiety to perform cell-specific targeting. There are three basic synthesis strategies for star polymers; first is the "core first" strategy where arms are coupled with the central multifunctional core. Second is the "coupling-onto" where conjugation reaction between a functional group and the preformed linear arm leads to a comb-shaped like structure. At last, comes the "arm-first" strategy in which covalent interactions are the factor behind attaching the arms with the core using crosslinking agents (Llewelyn et al. 2017; Schuetz et al. 2018).
Gaining the focus on resistant bacteria (ESKAPE bugs), incorporation of the antibiotic agent to these linear star polymers has resulted in a massive improvement in the antibacterial therapy. This includes attaching either the AMPs or antibacterial groups, which are polycationic, for e.g., poly(2-dimethylaminoethyl methacrylate), PDMAEMA based star polymers are susceptible to E. coli (99% in 2 h, MIC-less than 250 µg/ml) (Llewelyn et al. 2017; Schuetz et al. 2018). Studies related to the fact that the introduction of AMPs in these star polymers has proven that this process augments the characteristics of better encapsulation and compartmentalized functionalities of these star polymers, thus giving birth to the concept of stereospecific functionalized stars. Ring-opening polymerization (ROP) technique was adopted for the production of such star polymers. Stereospecific stars, also known as core-cross linked stars (CCS) were synthesized by ROP) of amino acid poly (ε-Z-L-lysine) N-carboxy anhydride (NCA), serving as the arm or the macromolecular initiator, followed by the addition of the cross-linking agent poly (L-cystine). Deprotection of the arms increased the water solubility of the CCS, which further increased the biocompatibility of the stars (Engler et al. 2011; Sulistio et al. 2011a; Wu et al. 2015; Huang et al. 2017).
Nanostructured antimicrobial peptide polymers
Colistin was the first antimicrobial agent preferred for the resistant Gram-negative bacteria Acinetobacter baumannii. However, due to the presence of an extra outer membrane layer and an additional defense mechanism of lipopolysaccharide, these ESKAPE bugs are becoming more and more dangerous and impossible to infiltrate. As mentioned earlier, AMPs incorporated as antimicrobial agents either as polycationic functional groups or amino acid sequences are found to be effective against these resistant microbes via electrostatic interactions but comes up with the adverse effect of toxicity (Sulistio et al. 2011b; Lam et al. 2014, 2016). Thus, exploring the strategy of nanostructured polymeric peptides or SNAPPs have shown excellent activity against both ESKAPE bugs as well as Colistin and MDR A. baumannii (CMDR). A mechanism involving outer membrane destabilization, initiation of an apoptotic cell death pathway, and disruption of ion movement across the cell membrane has established the antimicrobial efficacy of SNAPPs. In vitro antimicrobial tests indicated that even after 600 generations of S. aureus multiplication, any wild mutations were not observed even in the presence of sub-micron levels of SNAPPs prototype (S16) (Limmathurotsakul et al. 2019) revealing that these SNAPPs did not develop resistance.
Stereospecific structures of functional AMPs are commercially developed by using ROP-NCA (ring-opening polymerization N-carboxy anhydride) technique. In a recent study, SNAPPs were prepared using ROP-NCA which utilized lysine (cationic) and valine (hydrophobic) as amino acid residues. PAMAM dendritic arms using lysine to valine ratio of 2:1 to increase the water solubility of the structure were synthesized. The structures containing homolysine residues had a higher minimum bactericidal concentration (MBC) against E. coli. Furthermore, the antibacterial efficacy was found to be not species-specific. Localization of the charges owing to the nanostructure increases the bacterially induced peptide aggregation and thus increases the efficacy of the AMPs, which are formulated as SNAPPs. Unlike host defense peptides which only show bacterial pathway to directly prevent the activity of the ESKAPE bugs, SNAPPs show both bacterial as well as indirect pathway of immunizing the mammalian cells against CMDR and ESKAPE pathogens. The above mentioned indirect pathway is exhibited by increasing the neutrophil infiltration mechanism (O’Neill 2014; Lam et al. 2016).
Other strategies to develop AMPs are NCA-ROP techniques for utilizing alpha-amino acids. MRSA, P. aeruginosa, Serratia marcescens, and C. albicans (Devadas et al. 2019) were found to be highly susceptible at the lowest MIC when AMPs comprising of lysine (hydrophilic moiety), phenylalanine and leucine as the hydrophobic moiety in the ratio of 10:7.5:7.5 and lysine, phenylalanine in the ratio of 10:15 were used (Zhou et al. 2010; Raju 2011). Cationic polymers are preferred as they exhibit electrostatic interactions with the bacterial surface. Examples of certain synthesized cationic polymers include polyethyleneimines, polymethacrylates, polyarylamides, and protonated polyesters. Further, the development of polypeptide libraries by varying the carbon chain length of the side group functionalities gave an overall idea that they were effective against a broader spectrum of Gram-positive and Gram-negative bacterial populations and also prevent biofilm formation especially against E. coli and S. aureus (World Health Organization 2001; Engler et al. 2011).
Conclusion
A dearth of the antimicrobial agents has led to a major concern regarding infectious disease control. ESKAPE bugs have become self-sufficient in destroying every other antimicrobial delivery strategy. Numerous novel drug delivery systems that mimic the natural bacteriolytic action of peptides have been studied involving AMPs, incorporating those peptides as a nano-formulation and introduction into the star polymers. SNAPPs which describe the ultimate architecture of the star polymers have shown a promising future in combating AMR because of the additional apoptotic mechanism which is switched on by these SNAPPs once it gains access to the bacterial cell. Synthesis techniques of the AMPs have been in the limits of using ROP technique. However, extensive research in this field for the synthesis of AMPs to yield a cost-effective and reproducible outcome is very necessary. Modulation of the functionalities on the surface of the star polymers to check for a range of the therapeutic activity may be the futuristic goal for the upcoming research.
References
Almaaytah A, Mohammed GK, Abualhaijaa A, Al-Balas Q (2017) Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des Devel Ther 11:3159–3170. https://doi.org/10.2147/DDDT.S147450
Alvarez-Suarez J, Gasparrini M, Forbes-Hernández T et al (2014) The composition and biological activity of honey: a focus on Manuka honey. Foods 3:420–432. https://doi.org/10.3390/foods3030420
Arvizo R, Bhattacharya R, Mukherjee P (2010) Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin Drug Deliv 7:753–763. https://doi.org/10.1517/17425241003777010
Ashley EA, Recht J, Chua A et al (2018) An inventory of supranational antimicrobial resistance surveillance networks involving low- and middle-income countries since 2000. J Antimicrob Chemother 73:1737–1749. https://doi.org/10.1093/jac/dky026
Aslam B, Wang W, Arshad MI et al (2018) Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist 11:1645–1658. https://doi.org/10.2147/IDR.S173867
Australasian Science Magazine (2016) Polymer Stars Trump Antibiotic Resistance | Australasian Science Magazine. Australas. Sci
Boge L, Bysell H, Ringstad L et al (2016) Lipid-based liquid crystals as carriers for antimicrobial peptides: phase behavior and antimicrobial effect. Langmuir 32:4217–4228. https://doi.org/10.1021/acs.langmuir.6b00338
Brandelli A (2012) Nanostructures as promising tools for delivery of antimicrobial peptides. Mini-Rev Med Chem 12:731–741. https://doi.org/10.2174/138955712801264774
Braun K, Pochert A, Lindén M et al (2016) Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides. J Colloid Interface Sci 475:161–170. https://doi.org/10.1016/j.jcis.2016.05.002
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250. https://doi.org/10.1038/nrmicro1098
Carmona-Ribeiro AM, de Melo Carrasco LD (2014) Novel formulations for antimicrobial peptides. Int J Mol Sci 15:18040–18083. https://doi.org/10.3390/ijms151018040
Chen J, Wang F, Liu Q, Du J (2014) Antibacterial polymeric nanostructures for biomedical applications. Chem Commun 50:14482–14493. https://doi.org/10.1039/c4cc03001j
Chen WY, Chang HY, Lu JK et al (2015) Self-assembly of antimicrobial peptides on gold nanodots: against multidrug-resistant bacteria and wound-healing application. Adv Funct Mater 25:7189–7199. https://doi.org/10.1002/adfm.201503248
Chowdhury FR, Shariful Alam Jilani M, Barai L et al (2018) Melioidosis in Bangladesh: a clinical and epidemiological analysis of culture-confirmed cases. Trop Med Infect Dis. https://doi.org/10.3390/tropicalmed3020040
Cleophas RTC, Riool M, Quarles Van Ufford HC et al (2014) Convenient preparation of bactericidal hydrogels by covalent attachment of stabilized antimicrobial peptides using thiol-ene click chemistry. ACS Macro Lett 3:477–480. https://doi.org/10.1021/mz5001465
Dar OA, Hasan R, Schlundt J et al (2016) Exploring the evidence base for national and regional policy interventions to combat resistance. Lancet 387:285–295. https://doi.org/10.1016/S0140-6736(15)00520-6
Devadas SM, Nayak UY, Narayan R et al (2019) 2,5-Dimethyl-4-hydroxy-3(2H)-furanone as an anti-biofilm agent against non-Candida albicans Candida Species. Mycopathologia 184:403–411. https://doi.org/10.1007/s11046-019-00341-y
Dixit A, Kumar N, Kumar S, Trigun V (2019) Antimicrobial resistance: progress in the decade since emergence of New Delhi metallo-β-lactamase in India. Indian J Community Med 44:4–8. https://doi.org/10.4103/ijcm.IJCM_217_18
Engler AC, Shukla A, Puranam S et al (2011) Effects of side group functionality and molecular weight on the activity of synthetic antimicrobial polypeptides. Biomacromolecules 12:1666–1674. https://doi.org/10.1021/bm2000583
Fontela PS, O’Donnell S, Papenburg J (2018) Can biomarkers improve the rational use of antibiotics? Curr Opin Infect Dis 31:347–352. https://doi.org/10.1097/QCO.0000000000000467
Ghasemiyeh P, Mohammadi-Samani S (2019) Hydrogels as drug delivery systems; pros and cons. Trends Pharm Sci 5:7–24
Gruenheid S, Le Moual H (2012) Resistance to antimicrobial peptides in Gram-negative bacteria. FEMS Microbiol Lett 330:81–89. https://doi.org/10.1111/j.1574-6968.2012.02528.x
Hancock REW, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557. https://doi.org/10.1038/nbt1267
Hay SI, Rao PC, Dolecek C et al (2018) Measuring and mapping the global burden of antimicrobial resistance. BMC Med 16:78. https://doi.org/10.1186/s12916-018-1073-z
Huang HB, Peng JM, Weng L et al (2017) Procalcitonin-guided antibiotic therapy in intensive care unit patients: a systematic review and meta-analysis. Ann Intensive Care 7:114. https://doi.org/10.1186/s13613-017-0338-6
Kamaruzzaman NF, Tan LP, Hamdan RH et al (2019) Antimicrobial polymers: the potential replacement of existing antibiotics? Int J Mol Sci 20:2747. https://doi.org/10.3390/ijms20112747
Khan Yasmin, Nasar Muhammad Qasim, Numan Muhammad, Ullah Ikram. (2018) Biomimetic Synthesis of Silver Nanoparticles for Breast Cancer Therapeutics and Its Mechanism. Int J Nanotechnol Nanomedicine 3:
Lam SJ, O’Brien-Simpson NM, Pantarat N et al (2016) Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat Microbiol 1:16162. https://doi.org/10.1038/nmicrobiol.2016.162
Lam SJ, Sulistio A, Ladewig K et al (2014) Peptide-based star polymers as potential siRNA carriers. Aust J Chem 67:592. https://doi.org/10.1071/CH13525
Limmathurotsakul D, Sandoe JAT, Barrett DC et al (2019) “Antibiotic footprint” as a communication tool to aid reduction of antibiotic consumption. J Antimicrob Chemother 74:2122–2127. https://doi.org/10.1093/jac/dkz185
Llewelyn MJ, Fitzpatrick JM, Darwin E et al (2017) The antibiotic course has had its day. BMJ. https://doi.org/10.1136/bmj.j3418
Ma Y, Wang C, Li Y et al (2020) Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv Sci 7:1901872. https://doi.org/10.1002/advs.201901872
Mahlapuu M, Håkansson J, Ringstad L, Björn C (2016) Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194. https://doi.org/10.3389/fcimb.2016.00194
Makowski M, Silva ÍC, Do Amaral CP et al (2019) Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 11:588
Malakooti N, Alexander C, Alvarez-Lorenzo C (2015) Imprinted contact lenses for sustained release of polymyxin B and related antimicrobial peptides. J Pharm Sci 104:3386–3394. https://doi.org/10.1002/jps.24537
Martin-Serrano Á, Gómez R, Ortega P, La MFJD (2019) Nanosystems as vehicles for the delivery of antimicrobial peptides (Amps). Pharmaceutics 11:448. https://doi.org/10.3390/pharmaceutics11090448
Mendelson M, Balasegaram M, Jinks T et al (2017) Antibiotic resistance has a language problem. Nature 545:23–25. https://doi.org/10.1038/545023a
Mendelson M, Røttingen JA, Gopinathan U et al (2016) Maximising access to achieve appropriate human antimicrobial use in low-income and middle-income countries. Lancet 387:188–198. https://doi.org/10.1016/S0140-6736(15)00547-4
Mirjalili M, Zohoori S (2016) Review for application of electrospinning and electrospun nanofibers technology in textile industry. J Nanostructure Chem 6:207–213. https://doi.org/10.1007/s40097-016-0189-y
Mulani MS, Kamble EE, Kumkar SN et al (2019) Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: a review. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00539
Murali TS, Kavitha S, Spoorthi J et al (2014) Characteristics of microbial drug resistance and its correlates in chronic diabetic foot ulcer infections. J Med Microbiol 63:1377–1385. https://doi.org/10.1099/jmm.0.076034-0
Namivandi-Zangeneh R, Sadrearhami Z, Dutta D et al (2019) Synergy between synthetic antimicrobial polymer and antibiotics: a promising platform to combat multidrug-resistant bacteria. ACS Infect Dis 5:1357–1365. https://doi.org/10.1021/acsinfecdis.9b00049
Naveentaj S, Muzib YI (2020) A review on liquid crystalline nanoparticles (cubosomes): emerging nanoparticulate drug carrier. Int J Curr Pharm Res 12:5–9
NIH USNL of M ClinicalTrials.gov, NIH, U.S. National Library of Medicine. https://clinicaltrials.gov/. Accessed 26 May 2020
O’Neill JI (2014) Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist 20:1–6
Olesen SW, Barnett ML, Macfadden DR et al (2018) The distribution of antibiotic use and its association with antibiotic resistance. Elife 7:e39435. https://doi.org/10.7554/eLife.39435
Raju BBMBI (2011) A novel treatment approach towards emerging multidrug resistant Enteroaggregative Escherichia coli (EAEC) causing acute/persistent diarrhea using medicinal plant extracts—Manipal Academy of Higher Education, Manipal, India. Res J Pharm Biol Chem Sci 2:15–23
Rice LB (2008) Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197:1079–1081. https://doi.org/10.1086/533452
Ruden S, Hilpert K, Berditsch M et al (2009) Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob Agents Chemother 53:3538–3540. https://doi.org/10.1128/AAC.01106-08
Schuetz P, Wirz Y, Sager R et al (2018) Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis 18:95–107. https://doi.org/10.1016/S1473-3099(17)30592-3
Scorciapino MA, Serra I, Manzo G, Rinaldi AC (2017) Antimicrobial dendrimeric peptides: structure, activity and new therapeutic applications. Int J Mol Sci 18:542. https://doi.org/10.3390/ijms18030542
Shao C, Zhu Y, Lai Z et al (2019) Antimicrobial peptides with protease stability: progress and perspective. Future Med Chem 11:2047–2050. https://doi.org/10.4155/fmc-2019-0167
Shenoy V, Ballal M, Shivananda PG, Bairy I (2012) Honey as an antimicrobial agent against Pseudomonas aeruginosa isolated from infected wounds. J Glob Infect Dis 4:102–105. https://doi.org/10.4103/0974-777X.96770
Siriwardena TN, Capecchi A, Gan BH et al (2018) Optimizing antimicrobial peptide dendrimers in chemical space. Angew Chem Int Ed Engl 57:8483–8487. https://doi.org/10.1002/anie.201802837
Sulistio A, Lowenthal J, Blencowe A et al (2011a) Folic acid conjugated amino acid-based star polymers for active targeting of cancer cells. Biomacromol 12:3469–3477. https://doi.org/10.1021/bm200604h
Sulistio A, Widjaya A, Blencowe A et al (2011b) Star polymers composed entirely of amino acid building blocks: a route towards stereospecific, biodegradable and hierarchically functionalized stars. Chem Commun 47:1151–1153. https://doi.org/10.1039/c0cc03541f
Sun H, Hong Y, Xi Y et al (2018) Synthesis, self-assembly, and biomedical applications of antimicrobial peptide-polymer conjugates. Biomacromol 19:1701–1720. https://doi.org/10.1021/acs.biomac.8b00208
Tenover FC (2006) Mechanisms of antimicrobial resistance in bacteria. Am J Med 119:S3–S10. https://doi.org/10.1016/j.amjmed.2006.03.011
World Health Organization (2001) WHO Global Strategy for Containment of Antimicrobial Resistance
Wu W, Wang W, Li J (2015) Star polymers: Advances in biomedical applications. Prog Polym Sci 46:55–85. https://doi.org/10.1016/j.progpolymsci.2015.02.002
Yüksel E, Karakeçili A (2014) Antibacterial activity on electrospun poly(lactide-co-glycolide) based membranes via Magainin II grafting. Mater Sci Eng C 45:510–518. https://doi.org/10.1016/j.msec.2014.10.004
Zasloff M (2019) Antimicrobial peptides of multicellular organisms: my perspective. Adv Exp Med Biol 1117:3–6. https://doi.org/10.1007/978-981-13-3588-4_1
Zhou C, Qi X, Li P et al (2010) High potency and broad-spectrum antimicrobial peptides synthesized via ring-opening polymerization of α-Aminoacid-N-carboxyanhydrides. Biomacromol 11:60–67. https://doi.org/10.1021/bm900896h
Acknowledgements
Open access funding provided by Manipal Academy of Higher Education, Manipal. The authors would like to express their sincere gratitude to Manipal Academy of Higher Education, Manipal, for providing facilities for the literature search and facilities to work in this area. Authors are also thankful to the Indian Council of Medical Research (ICMR), New Delhi, Ref. No 2020–4549 for the fund support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mukhopadhyay, S., Bharath Prasad, A.S., Mehta, C.H. et al. Antimicrobial peptide polymers: no escape to ESKAPE pathogens—a review. World J Microbiol Biotechnol 36, 131 (2020). https://doi.org/10.1007/s11274-020-02907-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-020-02907-1