Abstract
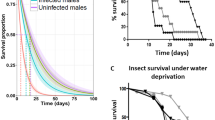
Male-killing, the death of male offspring induced by maternally transmitted microbes, is classified as early, or late, male-killing. The primary advantage afforded by early male-killing, which typically occurs during embryogenesis, is the reallocation of resources to females, that would have otherwise been consumed by males. Meanwhile, the key advantage of late male-killing, which typically occurs during late larval development, is the maximized potential for horizontal transmission. To date, no studies have reported on the associated developmental and physiological effects of host coinfection with early and late male-killers, which may have a significant impact on the population dynamics of the male-killers. Here we used a lepidopteran tea pest Homona magnanima as a model, which is a unique system wherein an early male-killer (a Spiroplasma bacterium) and a late male-killer (an RNA virus) can coexist in nature. An artificially established matriline, coinfected with both Spiroplasma and RNA virus, exhibited embryonic death (early male-killing) as seen in the host line singly infected with Spiroplasma. Moreover, the coinfected line also exhibited developmental retardation and low pupal weight similar to the host line singly infected with the RNA virus. A series of field surveys revealed that Spiroplasma-RNA virus coinfection occurs in nature at a low frequency. Hence, although the two male-killers are capable of coexisting within the H. magnanima population independently, high associated fitness cost appears to limit the prevalence of male-killer coinfection in the field host population.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Buchner P (1965) Endosymbiosis of animals with plant microorganisms. John Wiley & Sons Inc., New York
Bourtzis K, Miller TA (2003) Insect symbiosis. CRC Press, Florida
Bright M, Bulgheresi S (2010) A complex journey: transmission of microbial symbionts. Nat Rev Microbiol 8:218–230
Vavre F, Girin C, Boulétreau M (1999) Phylogenetic status of a fecundity-enhancing Wolbachia that does not induce thelytoky in Trichogramma. Insect Mol Biol 8:67–72
Fry AJ, Rand DM (2002) Wolbachia interactions that determine Drosophila melanogaster survival. Evolution 56:1976–1981
Brownlie JC, Cass BN, Riegler M, et al (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5:e1000368. https://doi.org/10.1371/journal.ppat.1000368
Duron O, Bouchon D, Boutin S, et al (2008) The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6:27 http://www.biomedcentral.com/1741-7007/6/27
Hurst GDD, Majerus MEN, Walker LE (1992) Cytoplasmic male killing elements in Adalia bipunctata (Linnaeus) (Coleoptera: Coccinellidae). Heredity 69:84–91
Majerus MEN, von der Schulenburg JHG, Zakharov IA (2000) Multiple causes of male-killing in a single sample of the two-spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae) from Moscow. Heredity 84:605–609
Andreadis TG, Hall DW (1979) Significance of transovarial infections of Amblyospora sp (Microspora: Thelohaniidae) in reaction to parasite maintenance in the mosquito Culex salinarius. J Invertebr Pathol 34:152–157
Sweeney AW, Hazard EI, Graham MF (1985) Intermediate host for an Amblyospora sp. (Microspora) infecting the mosquito, Culex annulirostris. J Invertebr Pathol 46:98–102
Morimoto S, Nakai M, Ono A, Kunimi Y (2001) Late male-killing phenomenon found in a Japanese population of the oriental tea tortrix, Homona magnanima (Lepidoptera: Tortricidae). Heredity 87:435–440
Nakanishi K, Hoshino M, Nakai M, Kunimi Y (2008) Novel RNA sequences associated with late male killing in Homona magnanima. P Roy Soc B-Biol Sci 275:1249–1254
Kageyama D, Yoshimura K, Sugimoto TN, et al (2017) Maternally transmitted non-bacterial male killer in Drosophila biauraria. Biol Lett 13:20170476. https://doi.org/10.1098/rsbl.2017.0476
Hurst GDD, Majerus MEN (1993) Why do maternally inherited microorganisms kill males? Heredity 71:81–95
Kondo N, Shimada M, Fukatsu T (2005) Infection density of Wolbachia endosymbiont affected by co-infection and host genotype. Biol Lett 1:488–491
Arai H, Hirano T, Akizuki N, et al (2019) Multiple infection and reproductive manipulations of Wolbachia in Homona magnanima (Lepidoptera: Tortricidae). Microb Ecol 77:257–266
Watanabe M, Miura K, Hunter MS, Wajnberg E (2011) Superinfection of cytoplasmic incompatibility-inducing Wolbachia is not additive in Orius strigicollis (Hemiptera: Anthocoridae). Heredity 106:642–648
Nakamura Y, Yukuhiro F, Matsumura M, Noda H (2012) Cytoplasmic incompatibility involving Cardinium and Wolbachia in the white-backed planthopper Sogatella furcifera (Hemiptera: Delphacidae). Appl Entomol Zool 47:273–283
Zhu LY, Zhang KJ, Zhang YK, et al (2012) Wolbachia strengthens Cardinium-induced cytoplasmic incompatibility in the spider mite Tetranychus piercei McGregor. Curr Microbiol 65:516–523
Goto S, Anbutsu H, Fukatsu T (2006) Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl Environ Microbiol 72:4805–4810
Teixeira L, Ferreira Á, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6:e2. https://doi.org/10.1371/journal.pbio.1000002
Hedges LM, Brownlie JC, O’Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322:702
Terradas G, McGraw EA (2017) Wolbachia-mediated virus blocking in the mosquito vector Aedes aegypti. Curr Opin Insect Sci 22:37–44
Hurst GDD, Jiggins FM, von der Schulenburg JHG et al (1999) Male–killing Wolbachia in two species of insect. P Roy Soc B-Biol Sci 266:735–740
Hurst GDD, von der Schulenburg JHG, Majerus TMO et al (1999) Invasion of one insect species, Adalia bipunctata, by two different male-killing bacteria. Insect Mol Biol 8:133–139
Werren JH, Hurst GDD, Zhang W, et al (1994) Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata). J Bacteriol 176:388–394
Hurst LD (1991) The incidences and evolution of cytoplasmic male killers. P Roy Soc B-Biol Sci 244:91–99
Majerus MEN, Hurst GDD (1997) Ladybirds as a model system for the study of male-killing symbionts. Entomophaga 42:13–20
Hurst GDD, Hurst LD, Majerus MEN (1997) Cytoplasmic sex-ratio distorters. In: O’Neill SL, Hoffmann AA, Werren JH (eds) Influential passengers. Oxford University Press, Oxford, pp 125–154
Arai H, Lin SR, Nakai M, et al (2019) Closely related male-killing and nonmale-killing Wolbachia strains in the oriental tea tortrix Homona magnanima. Microb Ecol:1–10
Tsugeno Y, Koyama H, Takamatsu T, et al (2017) Identification of an early male-killing agent in the oriental tea tortrix, Homona magnanima. J Hered 108:553–560
Hoshino M, Nakanishi K, Nakai M, Kunimi Y (2008) Gross morphology and histopathology of male-killing strain larvae in the oriental tea tortrix, Homona magnanima (Lepidoptera: Tortricidae). Appl Entomol Zool 43:119–125
Nishino M, Fujita R, Nakai M, et al (2018) Late male killing caused by novel RNA viruses Partitiviridae in a tea pest, Homona magnanima. Wolbachia 2018, Tenth International Wolbachia Conference, Salem MA, USA
Zhou W, Rousset F, O’Neil S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc Biol Sci 265:509–515
von der Schulenburg JHG, Majerus TMO, Dorzhu CM, et al (2000) Evolution of male-killing Spiroplasma (Procaryotae: Mollicutes) inferred from ribosomal spacer sequences. J Gen Appl Microbiol 46:95–98
Abràmoff MD, Magalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11:36–42
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4 arXiv preprint arXiv:1406.5823
Dunn AM, Smith JE (2001) Microsporidian life cycles and diversity: the relationship between virulence and transmission. Microbes Infect 3:381–388
Ahmed MZ, Li SJ, Xue X, et al (2015) The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog 11:e1004672
Jaenike J, Polak M, Fiskin A, et al (2007) Interspecific transmission of endosymbiotic Spiroplasma by mites. Biol Lett 3:23–25
Graham RI, Wilson K (2012) Male-killing Wolbachia and mitochondrial selective sweep in a migratory African insect. BMC Evol Biol 12:204
Nguyen DT, Morrow JL, Spooner-Hart RN, Riegler M (2017) Independent cytoplasmic incompatibility induced by Cardinium and Wolbachia maintains endosymbiont coinfections in haplodiploid thrips populations. Evolution 71:995–1008
Frank SA (1998) Dynamics of cytoplasmic incompatibility with multiple Wolbachia infections. J Theor Biol 192:213–218
Acknowledgments
We thank Y. Sato and C. Ishijima (National Agriculture and Food Research Organization, Shimada, Japan) for invaluable help in the field. We also thank Dr. H. Anbutsu (National Institute of Advanced Industrial Science and Technology, Tsukuba, Japan) for kind advice on our research. We also thank four anonymous reviewers for valuable suggestions.
Funding
This work was partially supported by the JSPS KAKENHI grant number JP24580076 and JSPS Research Fellowships for Young Scientists number 19J13123.
Author information
Authors and Affiliations
Contributions
TT conducted characterization of host lines and conducted field surveys. HA conducted field surveys, data analyses, and preparation for the manuscript. TT and HA contributed equally to the present work. NA established the host lines and conducted field surveys. MN supported entire experiments and contributed to discussion. YK supported the works, arranged surveys, and contributed to entire discussions of this study. MNI managed experiments and preparations for the manuscript and contributed to discussions of this study. Corresponding authors HA and MNI have full access to all data and had responsibility for the decision to submit for publication.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Electronic Supplementary Material
ESM 1
(DOCX 420 kb)
Rights and permissions
About this article
Cite this article
Takamatsu, T., Arai, H., Abe, N. et al. Coexistence of Two Male-Killers and Their Impact on the Development of Oriental Tea Tortrix Homona magnanima. Microb Ecol 81, 193–202 (2021). https://doi.org/10.1007/s00248-020-01566-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-020-01566-x