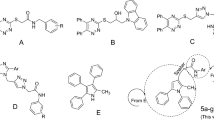
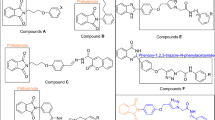
Abstract
A novel series of N-methyl/benzyl-substituted benzimidazolyl-linked para-substituted benzyl-based compounds containing 2,4-thiazolidinediones, dimethyl malonate (DMM), and diethyl malonate (DEM) 17–27 were designed, docked, synthesized, and evaluated for their antidiabetic activity studies. Structures of all the synthesized compounds were confirmed through 1H NMR, 13C NMR, FTIR, and mass spectrometry. Four targeted compounds (17–18 and 22–23) showed good inhibitory potential in the range of 4.10 ± 0.01 to 9.12 ± 0.06 µM. Furthermore, synthesized compounds 17–27 were evaluated for their antioxidant potential and compared with standard ascorbic acid and results showed that compound 18 (EC50 = 0.176 ± 0.002 mM) being the most active. Compounds 17–18 and 22–23 exhibited prominent antidiabetic as well as antioxidant activity. Compound 18 was considered a promising candidate for this series. The designed molecules were docked into α-glucosidase protein (PDB Code. 3TOP) to develop a correlation with the α-glucosidase inhibition studies and were also additionally docked into PPARγ proteins (PDB ID: 2PRG) with rosiglitazone (standard drug) to study their PPARγ binding affinity in comparison with rosiglitazone and to classify these compounds for their PPARγ agonistic behavior.












Similar content being viewed by others
References
Akhtar W, Khan MF, Verma G, Shaquiquzzaman M, Rizvi MA, Mehdi SH, Akhter M, Alam MM (2017) Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur J Med Chem 126:705–753
Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, Posner SF, Callaghan WM (2010) Diabetes trends among delivery hospitalizations in the U.S., 1994–2004. Diabetes Care 33:768–773
Arora RK, Kaur N, Bansal Y (2014) Novel coumarin–benzimidazole derivatives as antioxidants and safer anti-inflammatory agents. Acta Pharm Sin B 4:368–375
Arshad T, Khan KM, Rasool N, Salar U, Hussain S, Tahir T, Ashraf M, Wadood A, Riaz M, Perveen S, Taha M, Ismail NH (2016) Syntheses, in vitro evaluation and molecular docking studies of 5-bromo-2-aryl benzimidazoles as α-glucosidase inhibitors. Med Chem Res 25:2058–2069
Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53:409–435
Bruning JB, Chalmers MJ, Prasad S, Busby SA, Kamenecka TM, He Y, Nettles KW, Griffin PR (2007) Partial agonists activate PPARγ using a helix 12 independent mechanism. Structure 15:1258–1271
Carmona MC, Louche K, Lefebvre B, Pilon A, Hennuyer N, Bouchez VA, Fievet C, Torpier G, Formstecher P, Renard P, Lefebvre P, Dacquet C, Staels B, Casteilla L, Penicaud L (2007) S 26948: a new specific peroxisome proliferator–activated receptor modulator with potent antidiabetes and antiatherogenic effects. Diabetes 56:2797–2808
Ceriell A (2000) Oxidative stress and glycemic regulation. Metabolism 49:27–29
Chakrabarti R, Rajagopalan R (2002) Diabetes and insulin resistance associated disorders: disease and the therapy. Curr Sci 83:1533–1538
Chinthala Y, Domatti AK, Sarfaraz A, Singh SP, Arigari NK, Gupta N, Satya SKVN, Kumar JK, Khan F, Tiwari AK, Paramjit G (2013) Synthesis, biological evaluation and molecular modeling studies of some novel thiazolidinediones with triazole ring. Eur J Med Chem 70:308–314
Dhameja M, Gupta P (2019) Synthetic heterocyclic candidates as promising α-glucosidase inhibitors: an overview. Eur J Med Chem 176:343–377
Dinparast L, Valizadeh H, Bahadori MB, Soltani S, Asghari B, Rashidi MR (2016) Design, synthesis, α-glucosidase inhibitory activity, molecular docking and QSAR studies of benzimidazole derivatives. J Mol Struct 1114:84–94
Einstein M, Akiyama TE, Castriota GA, Wang CF, McKeever B, Mosley RT, Becker JW, Moller DE, Meinke PT, Wood HB, Berger JP (2008) The differential interactions of peroxisome proliferatoractivated receptor ligands with TYR473 is a physical basis for their unique biological activities. Mol Pharm 73:62–74
Ghani U (2015) Re-exploring promising α-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur J Med Chem 103:133–162
Granfeldt Y, Björck I, Drews A, Tovar J (1992) An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. Eur J Clin Nutr 46:649–660
Gray GM (1975) Carbohydrate digestion and absorption—role of the small intestine. N Eng J Med 292:1225–1230
Guasch L, Sala E, Valls C, Blay M, Mulero M, Arola L, Pujadas G, Vallvee SG (2011) Structural insights for the design of new PPARgamma partial agonists with high binding affinity and low transactivation activity. J Comput Aided Mol Des 25:717–728
Hirose H, Yamasaki T, Ogino M, Mizojiri R, Tamura OY, Yashiro H, Muraki Y, Nakano Y, Sugama J, Hata A, Iwasaki S, Watanabe M, Maekawa T, Kasai S (2017) Discovery of novel 5-oxa-2,6-diazaspiro[3.4]oct-6-ene derivatives as potent, selective, and orally available somatostatin receptor subtype 5(SSTR5) antagonists for treatment of type 2 diabetes mellitus. Bioorg Med Chem 25:4175–4193
Ibrahim MK, Eissa IH, Abdallah AE, Metwaly AM, Radwan MM, ElSohly MA (2017) Design, synthesis, molecular modeling and anti-hyperglycemic evaluation of novel quinoxaline derivatives as potential PPARγ and SUR agonists. Bioorg Med Chem 25:1496–1513
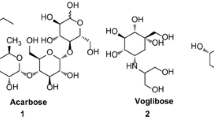
Kaku K (2014) Efficacy of voglibose in type 2 diabetes. Expert Opin Pharm 15:1181–1190
Kaur J, Singh A, Singh G, Verma RK, Mall R (2018) Novel indolyl linked para-substituted benzylidene-based phenyl containing thiazolidienediones and their analogs as α-glucosidase inhibitors: synthesis, in vitro, and molecular docking studies. Med Chem Res 27:903–914
Kim T, Choi HJ, Eom SH, Lee J, Kim TH (2014) Potential α-glucosidase inhibitors from thermal transformation of (±)-catechin. Bioorg Med Chem Lett 24:1621–1624
Liu W, Lau F, Liu K, Wood HB, Zhou G, Chen Y, Li Y, Akiyama TE, Castriota G, Einstein M, Wang C, McCann ME, Doebber TW, Wu M, Chang CH, McNamara L, McKeever B, Mosley RT, Berger JP, Meinke PT (2011) Benzimidazolones: a new class of selective peroxisome proliferator activated receptor γ (PPARγ) modulators. J Med Chem 53:8541–8554
Mall R, Singh A, Singh G, Singh V, Verma RK (2019) Indolyl linked meta-substituted benzylidenes as novel ligands: synthesis, biological evaluation, and molecular docking studies. J Heterocycl Chem 56:1542–1555
Mavrova AT, Yancheva D, Anastassova N, Anichina K, Zvezdanovic J, Djordjevic A, Markovic D, Smelcerovic A (2015) Synthesis, electronic properties, antioxidant and antibacterial activity of some new benzimidazoles. Bioorg Med Chem 23:6317–6326
Menteşe E, Ulker S, Kahveci B (2015) Synthesis and study of α-glucosidase inhibitory, antimicrobial and antioxidant activities of some benzimidazole derivatives containing triazole, thiadiazole, oxadiazole, and morpholine rings. Chem Heterocycl Compd 5:1671–1685
Mizuno CS, Chittiboyina AG, Kurtz TW, Pershadsingh HA, Avery MA (2008) Type 2 diabetes and oral antihyperglycemic drugs. Curr Med Chem 15:61–74
Musialik M, Kuzmicz R, Pawłowski TS, Litwinienko G (2009) Acidity of hydroxyl groups: an overlooked influence on antiradical properties of flavonoids. J Org Chem 74:2699–2709
Musialik M, Litwinienko G (2005) Scavenging of dpph• radicals by vitamin E is accelerated by its partial ionization: the role of sequential proton loss electron transfer. Org Lett 7:4951–4954
Najafi M, Najafi M, Najafi H (2012) DFT/B3LYP study to investigate the possible ways for the synthesize of antioxidants with high efficiency based on vitamin E. Bull Korean Chem Soc 33:3343–3348
Notkins AL (2002) Immunologic and genetic factors in Type 1 diabetes. J Biol Chem 277:43545–43548
Ozil M, Emirik M, Belduz A, Ulker S (2016a) Molecular docking studies and synthesis of novel bisbenzimidazole derivatives as inhibitors of a-glucosidase. Bioorg Med Chem 24:5103–5114
Ozil M, Emirik M, Etlik SY, Ulke S, Kahveci B (2016b) A simple and efficient synthesis of novel inhibitors of alpha-glucosidase based on benzimidazole skeleton and molecular docking studies. Bioorg Chem 68:226–235
Özil M, Parlak C, Baltaş N (2016c) A simple and efficient synthesis of benzimidazoles containing piperazine or morpholine skeleton at C-6 position as glucosidase inhibitors with antioxidant activity. Bioorg Chem 76:468–477
Rekha S, Chandrashekhara S (2015) Antioxidant and antibacterial activities of thiazolidinedionederivatives. IJPSR 1:421–428
Scott LJ, Spencer CM (2000) Miglitol a review of its therapeutic potential in type 2 diabetes mellitus. Drugs 59:521–549
Singh A, Verma RK, Kuhad A, Mall R (2017) Novel indole-2-carboxylic acid linked 3-phenyl-2-alkoxy propanoic acids: synthesis, molecular docking and in vivo antidiabetic studies. Med Chem Res 26:745–759
Singh G, Singh A, Verma RK, Mall R, Azeem U (2018a) Synthesis, biological evaluation and molecular docking studies of novel benzimidazole derivatives. Comput Biol Chem 72:45–52
Singh V, Singh A, Singh G, Verma RK, Mall R (2018b) Novel benzoxazole derivatives featuring rhodanine and analogs as antihypergycemic agents: synthesis, molecular docking, and biological studies. Med Chem Res 27:735–743
Shahidpour S, Panahi F, Yousefi R, Nourisefat M, Nabipoor M, Nezhad AK (2015) Design and synthesis of new antidiabetic α-glucosidase and α-amylase inhibitors based on pyrimidine-fused heterocycles. Med chem Res 24:3086–3096
Tahaa M, Ismail NH, Imran S, Mohamad MH, Wadood A, Rahim F, Saad SM, Rehman A, Khan KM (2016) Synthesis, α-glucosidase inhibitory, cytotoxicity and docking studies of 2-aryl-7-methylbenzimidazoles. Bioorg Chem 65:100–109
Vallvee SG, Guasch L, Hernández ST, Bas JM, Ollendorff V, Arola L, Pujadas G, Mulero M (2015) Peroxisome proliferator-activated receptor γ (PPARγ) and ligand choreography: newcomers take the stage. J Med Chem 58:5381–5394
Verma RK, Ghosh P, Kumar V, Wadhwa LK (2012a) The application of comparative molecular field analysis for the design of α-anilino substituted-3-phenyl propanoic acids as novel PPARα/γ dual ligands. Med Chem Res 21:2873–2884
Verma RK, Kumar V, Ghosh P, Wadhwa LK (2012b) Heterocyclyl linked anilines and benzaldehydes as precursors for biologically significant new chemical entities. J Chem Sci 124:1063–1069
Verma RK, Kumar V, Ghosh P, Wadhwa LK (2013b) 3D-QSAR study of tyrosine and propanoic acid derivatives as PPARα/γ dual agonists using CoMSIA. Med Chem Res 22:287–302
Verma RK, Mall R, Ghosh P, Kumar V(2013) Design and synthesis of benzimidazole-linked meta-substituted benzylidenes/benzyls as biologically significant new chemical entities Synth Commun 43:1882–1895
Verma RK, Mall R, Singh A (2015) Indolyl linked meta-substituted benzylidene-based novel PPAR ligands: synthetic and docking studies. Med Chem Res 24:1396–1407
Wang G, Chen M, Wang J, Peng Y, Li L, Xie ZZ, Deng B, Chen S, Li W (2017a) Synthesis, biological evaluation and molecular docking studies of chromone hydrazone derivatives as α-glucosidase inhibitors. Bioorg Med Chem Lett 27:2957–2961
Wang G, Peng Y, Xie Z, Wang J, Chen M (2017b) Synthesis, α-glucosidase inhibition and molecular docking studies of novel thiazolidine-2,4-dione or rhodanine derivatives. Med Chem Commun 8:1477–1484
West IC (2000) Radicals and oxidative stress in diabetes. Diabet Med 17:171–180
Yee HS, Fong NT (1996) A review of the safety and efficacy of Acarbose in diabetes mellitus. Pharmacotherapy 16:792–805
Yousefi A, Yousefi R, Panahi F, Sarikhani S, Zolghadr A, Bahaoddini A, Nezhad AK (2015) Novel curcumin-based pyrano[2,3-d]pyrimidine anti-oxidant inhibitors for α-amylase and α-glucosidase: implications for their pleiotropic effects against diabetes complications. Int J Biol Macromol 78:46–55
Zawawi NKNA, Taha M, Ahmat N, Ismail NH, Wadood A, Rahim F (2017) Synthesis, molecular docking studies of hybrid benzimidazole as α-glucosidase inhibitor. Bioorg Chem 70:184–191
Zawawi NKNA, Taha M, Ahmat N, Wadood A, Ismail NH, Rahim F, Azam SS, Abdullah N (2016) Benzimidazole derivatives as new α-glucosidase inhibitors and in silico studies. Bioorg Chem 64:29–36
Zhang B, Xing Y, Wen C, Yu X, Sun W, Xiu Z, Dong Y (2017) Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: structure-activity relationships and the synergism with acarbose. Bioorg Med Chem Lett 27:5065–5070
Acknowledgements
Authors are thankful to the Punjabi University, Patiala authorities for providing the necessary research facilities. We are also grateful to Director and Mr Avtar Singh of Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh, respectively, for extending the facilities for spectral analysis of the compounds reported in this paper. One of the authors, GS, is thankful to the Ministry of Social Justice and Empowerment, Govt of India for providing research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Singh, G., Singh, A., Singh, V. et al. Synthesis, molecular docking, α-glucosidase inhibition, and antioxidant activity studies of novel benzimidazole derivatives. Med Chem Res 29, 1846–1866 (2020). https://doi.org/10.1007/s00044-020-02605-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02605-5