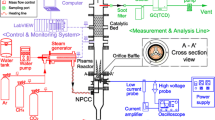
Abstract
Spent petrochemical catalysts are hazardous toxic wastes and dangerous to the environment and human health, due to heavy metals, coke, and other poisonous elements. Researchers over the years focus to utilize and handle the spent catalysts waste to produce other valuable products as an attractive option from environmental and economic points of view. This article generally discusses feasible methods to convert spent petrochemical waste catalysts to value-added products using a thermal plasma torch for the first time. The arc temperature which increases with power increase was measured by optical emission spectroscopy (OES). The result reveals that no spent catalyst waste remains after plasma pyrolysis process and conversion is completely feasible. Furthermore, the major product H2 for fuel cell was produced, that is environmentally and economically beneficial. Methane, ethane, ethylene, and isobutane were the main products. The best plasma effect on the spent petrochemical catalyst waste in terms of the maximum hydrogen production rate was obtained at 140 A, 30 V, and 3 cc feed injection which causes to produce 53.2% H2.











Similar content being viewed by others
References
Dou S et al. (2018) Plasma‐assisted synthesis and surface modification of electrode materials for renewable energy. Adv Mater 1705850
Bayatsarmadi B et al (2017) Recent advances in atomic metal doping of carbon-based nanomaterials for energy conversion. Small 13(21):1700191
Lee DU et al (2016) Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal–air batteries. J Mater Chem A 4(19):7107–7134
Jun Li KL, Shengjun Y, Yaojian L, Dan H (2016) Application of thermal plasma technology for the treatment of solid wastes in China: an overview. Waste Manag 58:260–269
Khani M et al (2014) Effect of microwave plasma torch on the pyrolysis fuel oil in the presence of methane and ethane to increase hydrogen production. Int J Hydrogen Energy 39(33):18812–18819
Xin-gang Z et al (2016) Technology, cost, a performance of waste-to-energy incineration industry in China. Renew Sustain Energy Rev 55:115–130
Zhao X-G et al (2016) Economic analysis of waste-to-energy industry in China. Waste Manag 48:604–618
Sánchez-García M et al (2015) Biochar accelerates organic matter degradation and enhances N mineralisation during composting of poultry manure without a relevant impact on gas emissions. Biores Technol 192:272–279
Ojha A, Reuben AC, Sharma D (2012) Solid waste management in developing countries through plasma arc gasification-an alternative approach. APCBEE Procedia 1:193–198
Van der Walt I et al (2012) An economic evaluation for small scale thermal plasma waste-to-energy systems. In: Proceedings of the international plasma chemistry society, vol 21
Williams R, Jenkins B, Nguyen D (2003) Solid waste conversion: A review and database of current and emerging technologies. Final report, Department of Biological and Agricultural Engineering, University of California, Davis
Duan S et al (2017) Plasma surface modification of materials and their entrapment of water contaminant: a review. Plasma Process Polym 14(9):1600218
Ruj B, Ghosh S (2014) Technological aspects for thermal plasma treatment of municipal solid waste—a review. Fuel Process Technol 126:298–308
Gomez E et al (2009) Thermal plasma technology for the treatment of wastes: a critical review. J Hazard Mater 161(2–3):614–626
Desmet T et al (2009) Nonthermal plasma technology as a versatile strategy for polymeric biomaterials surface modification: a review. Biomacromol 10(9):2351–2378
Chu PK et al (2002) Plasma-surface modification of biomaterials. Mater Sci Eng R Rep 36(5–6):143–206
Bogaerts A et al (2002) Gas discharge plasmas and their applications. Spectrochim Acta, Part B 57(4):609–658
Kizling MB, Järås SG (1996) A review of the use of plasma techniques in catalyst preparation and catalytic reactions. Appl Catal A 147(1):1–21
Huang H, Tang L (2007) Treatment of organic waste using thermal plasma pyrolysis technology. Energy Convers Manag 48(4):1331–1337
Bonizzoni G, Vassallo E (2002) Plasma physics and technology; industrial applications. Vacuum 64(3–4):327–336
Agon N et al (2016) Plasma gasification of refuse derived fuel in a single-stage system using different gasifying agents. Waste Manag 47:246–255
Materazzi M et al (2016) Performance analysis of RDF gasification in a two stage fluidized bed–plasma process. Waste Manag 47:256–266
Materazzi M et al (2013) Thermodynamic modelling and evaluation of a two-stage thermal process for waste gasification. Fuel 108:356–369
Wang Q et al (2010) Application of thermal plasma to vitrify fly ash from municipal solid waste incinerators. Chemosphere 78(5):626–630
Basu P (2006) Combustion and gasification in fluidized beds. CRC Press, USA
Tang L, Huang H (2004) An investigation of sulfur distribution during thermal plasma pyrolysis of used tires. J Anal Appl Pyrol 72(1):35–40
Gandhi H (2015) Plasma gasification: from a dirty city to a heavenly place and from waste solids to clean fuel. Int J Innov Res Sci Technol 1:18–24
Fabry F et al (2013) Waste gasification by thermal plasma: a review. Waste Biomass Valorization 4(3):421–439
Yang L et al (2011) Solid waste plasma disposal plant. J Electrostat 69(5):411–413
Young GC (2010) Municipal solid waste to energy conversion processes: economic, technical, and renewable comparisons. Wiley, USA
Murphy A (1999) Plasma destruction of gaseous and liquid wastes. Ann N Y Acad Sci 891(1):106–123
Heberlein J, Murphy AB (2008) Thermal plasma waste treatment. J Phys D Appl Phys 41(5):053001
Pacheco J et al (2001) Recovering energetic gas from hydrocarbon solutions treated by thermal plasma. In: Proceedings of 25th international conference on phenomena in ionized gases
Kim S-W, Park H-S, Kim H-J (2003) 100 kW steam plasma process for treatment of PCBs (polychlorinated biphenyls) waste. Vacuum 70(1):59–66
Burkhard R, Hoffelner W, Eschenbach R (1994) Recycling of metals from waste with thermal plasma. Resour Conserv Recycl 10(1–2):11–16
Wong FF et al (2006) Recovery and reduction of spent nickel oxide catalyst via plasma sintering technique. Plasma Chem Plasma Process 26(6):585–595
Chiang K-C et al (2011) Recovery of spent alumina-supported platinum catalyst and reduction of platinum oxide via plasma sintering technique. J Taiwan Inst Chem Eng 42(1):158–165
Marafi M, Rana M (2016) Refinery waste: the spent hydroprocessing catalyst and its recycling options. In: 8th international conference on waste management and the environment (proceedings)
Khosravi A et al (2018) The processing of pyrolysis fuel oil by dielectric barrier discharge plasma torch. Plasma Chem Plasma Process 38(2):365–378
Gharibi M et al (2015) Dielectric barrier discharge plasma torch treatment of pyrolysis fuel oil in presence of methane and ethane. J Electrostat 76:178–187
Khani MR et al (2015) Conversion of pyrolysis fuel oils by a dielectric barrier discharge reactor in the presence of methane and ethane. Chem Eng Technol 38(8):1452–1459
Khani MR et al (2014) Study on the feasibility of plasma (DBD Reactor) cracking of different hydrocarbons (n-hexadecane, lubricating oil, and heavy oil). IEEE Trans Plasma Sci 42(9):2213–2220
Khani M et al (2014) The effects of microwave plasma torch on the cracking of pyrolysis fuel oil feedstock. Chem Eng J 237:169–175
Auciello O, Flamm DL (2013) Plasma diagnostics: discharge parameters and chemistry, Academic Press, USA
Kunze H-J (2009) Introduction to plasma spectroscopy, Springer Science & Business Media, Berlin
Radziemski LJ, Cremers DA (2006) Handbook of laser induced breakdown spectroscopy, Wiley, USA, pp 1–4
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karimi, H., Khani, M.R., Gharibi, M. et al. Plasma pyrolysis feasibility study of spent petrochemical catalyst wastes to hydrogen production. J Mater Cycles Waste Manag 22, 2059–2070 (2020). https://doi.org/10.1007/s10163-020-01089-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-020-01089-0