Abstract
This study demonstrates the design, synthesis and sensing applications of a simple and efficient chemosensor, 2-(1,3-dioxo-1H-inden-2(3H)-ylidene)hydrazinecarbothioamide (R) for quick detection of Hg2+ and F− in aqueous media using a colorimetric test, UV–Vis spectral analysis, and silica gel support. Sensor R showed the typical absorption peak at 305 nm for Hg2+ which is responsible for color change from yellow to colorless, while in the case of F−, R exhibits a major peak at 415 nm and a color change from yellow to red. The limit of detection (LOD) of R for the analysis of Hg2+ was calculated as 1×10−6 M while for F−, it was found to be 3.4×10−7 M. The binding modes of R with Hg2+ and F− have been investigated by the Job’s plot, Benesi–Hildebrand (BH) method, 1H NMR, FTIR and theoretical studies. The sensor R was reversible after treating it with reagents such as EDTA and calcium nitrate solution after contacting with Hg2+ and F−, respectively. The performance of R can be successfully applied for the analysis of F− contents present in toothpastes.
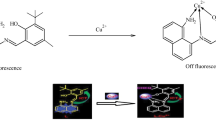
Graphic abstract
A simple and efficient receptor ninhydrin-based Schiff’s base (R) for quick detection of Hg2+ and F− in aqueous medium using colorimetric and UV–Vis spectral analyses.
















Similar content being viewed by others
References
Aragay G, Pons J and Merkoçi A 2011 Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection Chem. Rev. 111 3433
Carter K P, Young A M and Palmer A E 2014 Fluorescent sensors for measuring metal ions in living systems Chem. Rev. 114 4564
Cho D G and Sessler J L 2009 Modern reaction-based indicator systems Chem. Soc. Rev. 38 1647
Kaur K, Saini R, Kumar A, Luxami V, Kaur N, Singh P and Kumar S 2012 Chemodosimeters: an approach for detection and estimation of biologically and medically relevant metal ions, anions and thiols Coord. Chem. Rev. 256 1992
Santos-Figueroa L E, Moragues M E, Climent E, Agostini A, Martínez-Máñez R and Sancenon F 2013 Chromogenic and fluorogenic chemosensors and reagents for anions. A comprehensive review of the years 2010–2011 Chem. Soc. Rev. 42 3489
Sareen D, Kaur P and Singh K 2014 Strategies in detection of metal ions using dyes Coord. Chem. Rev. 265 125
Xu Z, Chen X, Kim H N and Yoon J 2010 Sensors for the optical detection of cyanide ion Chem. Soc. Rev. 39 127
Formica M, Fusi V, Giorgi L and Micheloni M 2012 New fluorescent chemosensors for metal ions in solution Coord. Chem. Rev. 256 170
Gai L, Mack J, Lu H, Nyokong T, Li Z, Kobayashi N and Shen Z 2012 Organosilicon compounds as fluorescent chemosensors for fluoride anion recognition Coord. Chem. Rev. 285 24
Kim H N, Ren W X, Kim J S and Yoon J 2012 Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions Chem. Soc. Rev. 41 3210
Nolan E M and Lippard S J 2008 Tools and tactics for the optical detection of mercuric ion Chem. Rev. 108 3443
Yang Y, Zhao Q, Feng W and Li F 2012 Luminescent chemodosimeters for bioimaging Chem. Rev. 113 192
Zhou Y, Zhang J F and Yoon J 2014 Fluorescence and colorimetric chemosensors for fluoride-ion detection Chem. Rev. 114 5511
Ayoob S and Gupta A K 2006 Fluoride in drinking water: a review on the status and stress effects Crit. Rev. Environ. Sci. Technol. 36 433
Cametti M and Rissanen K 1983 Recognition and sensing of fluoride anion Chem. Commun. 20 2809
Wergedal J E and Baylink D J Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells Science 222 330
Kleerekoper M The role of fluoride in the prevention of osteoporosis Endocrinol. Metab. Clin. 27 441
Yamaguchi S, Akiyama S and Tamao K Colorimetric fluoride ion sensing by boron-containing π-electron systems J. Am. Chem. Soc. 123 11372
Cittanova M L, Lelongt B, Verpont M C, Geniteau-Legendre M, Wahbe F, Prie D, Coriat P and Ronco P M 1996 Fluoride ion toxicity in human kidney collecting duct cells Anesthesiology 84 428
Kim S Y, Park J, Koh M, Park S B and Hong J I 2009 Fluorescent probe for detection of fluoride in water and bioimaging in A549 human lung carcinoma cells Chem. Commun. 31 4735
Singh P, Barjatiya M, Dhing S, Bhatnagar R, Kothari S and Dhar V 2001 Evidence suggesting that high intake of fluoride provokes nephrolithiasis in tribal populations Urol. Res. 29 238
Wang C and Wong K M C 2013 Selective Hg2+ sensing behaviors of rhodamine derivatives with extended conjugation based on two successive ring-opening processes Inorg. Chem. 52 13432
He S, Liu Q, Li Y, Wei F, Cai S, Lu Y and Zeng X 2014 Rhodamine 6G-based chemosensor for the visual detection of Cu2+ and fluorescent detection of Hg2+ in water Chem. Res. Chinese Univ. 30 32
Khatua S and Schmittel M 2013 A single molecular light-up sensor for quantification of Hg2+ and Ag+ in aqueous medium: high selectivity toward Hg2+ over Ag+ in a mixture Org. Lett. 15 4422
Parthiban C, Manivannan R and Elango K P 2015 Highly selective colorimetric sensing of Hg (II) ions in aqueous medium and in the solid state via formation of a novel M–C bond Dalt. Trans. 44 3259
Varghese A and George L 2012 Simultaneous first order derivative spectrophotometric determination of vanadium and zirconium in alloy steels and minerals Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 95 46
Easmon J, Pürstinger G, Heinisch G, Roth T, Fiebig H H, Holzer W, Jäger W, Jenny M and Hofmann J 2001 Synthesis, cytotoxicity, and antitumor activity of copper (II) and iron (II) complexes of 4 N-azabicyclo[3.2.2]nonane thiosemicarbazones derived from acyl diazines J. Med. Chem. 44 2164
Chellan P, Nasser S, Vivas L, Chibale K and Smith G S 2010 Cyclopalladated complexes containing tridentate thiosemicarbazone ligands of biological significance: Synthesis, structure and antimalarial activity J. Organomet. Chem. 695 2225
Calatayud D G, López Torres E and Mendiola M A 2013 A fluorescent dissymmetric thiosemicarbazone ligand containing a hydrazonequinoline arm and its complexes with cadmium and mercury Eur. J. Inorg. Chem. 1 80
Su H, Huang W, Yang Z, Lin H and Lin H 2012 2-Hydroxy-naphth-1-aldehyde phenyl-thiosemicarbazone: effective thiourea-based sensor for acetate anion J. Incl. Phenom. Macrocycl. Chem. 72 221
Raju V, Ashok Kumar S K, Abbareddy D S, Rao M and Sahoo S K 2017 Isatin-3-phenylhydrazone: a highly selective colorimetric chemosensor for copper, chromium and cobalt ions in semi-aqueous medium Sens. Lett. 15 266
West D X, Liberta A E, Padhye S B, Chikate R C, Sonawane P B, Kumbhar A S and Yerande R G 1993 Thiosemicarbazone complexes of copper(II): structural and biological studies, Coord. Chem. Rev. 123 49
Tian Y P, Duan C Y, Lu Z L, You X Z, Fun H K and Kandasamy S 1996 Crystal structure and spectroscopic studies on metal complexes containing ns donor ligands derived from S-benzyldithiocarbazate and p-dimethylaminobenzaldehyde Polyhedron. 15 2263
Souza P, Matesanz A I and Fernández V 1996 Copper (II) and cobalt (II) complexes of methyl 2-pyridyl ketone thiosemicarbazone (HL); single-crystal structure of [Cu (HL) L] NCS J. Chem. Soc. Dalt. Trans. 14 3011
Lu Z, White C, Rheingold A L and Crabtree R H 1993 Deprotonated thioamides as thiolate S-donor ligands with a high tendency to avoid MSM bridge formation: crystal and molecular structure of bis(2-hydroxy-5-methylacetophenone N,N-dimethylthiosemicarbazonato)dinickel Inorg. Chem. 32 3991
Kumar R R, Ramesh R and Małecki J G 2016 Steric control on the coordination behaviour of carbazole thiosemicarbazones towards [RuH (Cl)(CO)(AsPh 3)3]: a combined experimental and theoretical study New J. Chem. 40 10084
Frisch M J et al. 2009 Gaussian 09, Revision A.02 (Wallingford, CT: Gaussian, Inc.)
Wu C, Wang J, Shen J, Bi C and Zhou H 2017 Coumarin-based Hg2+ fluorescent probe: Synthesis and turn-on fluorescence detection in neat aqueous solution Sensors Actuators B Chem. 243 678
Wang J, Li W and Long L 2017 Development of a near-infrared fluorescence turn-on probe for imaging Hg2+ in living cells and animals Sensors Actuators B Chem. 245 462
Guo Z, Zhu W, Zhu M, Wu X and Tian H 2010 Near infrared cell permeable Hg2+ selective ratiometric fluorescent chemodosimeters and fast indicator paper for MeHg+ based on tricarbocyanines Chem. Eur. J. 16 14424
Momidi B K, Tekuri V and Trivedi D R 2016 Selective detection of mercury ions using benzothiazole based colorimetric chemosensor Inorg. Chem. Commun. 74 1
Pan J T, Zhu F, Kong L, Yang L M, Tao X T, Tian Y P, Lu H B and Yang J X 2015 A simple pyridine-based colorimetric chemosensor for highly sensitive and selective mercury (II) detection with the naked eye Chem. Pap. 69 527.
Ma L, Leng T, Wang K, Wang C, Shen Y and Zhu W 2017 A coumarin-based fluorescent and colorimetric chemosensor for rapid detection of fluoride ion Tetrahedron 73 1306
Chavali R, Gunda N S K, Naicker S and Mitra S K 2015 Rapid detection of fluoride in potable water using a novel fluorogenic compound 7-O-tert-butyldiphenylsilyl-4-methylcoumarin Anal. Chem. Res. 6 26
Mohammadi A and Jabbari J 2016 Simple naked-eye colorimetric chemosensors based on Schiff-base for selective sensing of cyanide and fluoride ions Can. J. Chem. 94 631
Chen Z J, Wang L M, Zou G, Zhang L, Zhang G J, Cai X F and Teng M S 2012 Colorimetric and ratiometric fluorescent chemosensor for fluoride ion based on perylene diimide derivatives Dye. Pigment. 94 410
Vinithra G, Suganya S and Velmathi S 2013 Naked eye sensing of anions using thiourea based chemosensors with real time application Tetrahedron Lett. 54 5612
Acknowledgements
Authors are grateful to the Department of Science and Technology, GOI for supporting the work through the project grant (EMR/2017/000816). Authors are also thankful to DST-VIT-FIST for NMR and GC-MS for research facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Raju, V., Kumar, R.S., Kumar, S.K.A. et al. A ninhydrin–thiosemicarbazone based highly selective and sensitive chromogenic sensor for Hg2+ and F− ions. J Chem Sci 132, 89 (2020). https://doi.org/10.1007/s12039-020-01799-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-01799-w