Abstract
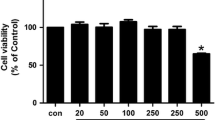
Tetramerized single-chain variable fragment (ScFv) of anti-cyclic citrullinated peptide (TeAb-CCP) is a constructed tetramerized ScFv of anti-cyclic citrullinated peptide (CCP) antibodies with p53 tetrameric domain, aim to investigate its effect on fibroblast-like synoviocytes (FLSs) proliferation, migration, invasion, and production of inflammatory mediators in the in vitro co-culture system of peripheral mononuclear cells (PBMCs) and FLSs. TeAb-CCP was constructed by modifying a monovalent ScFv antibody to CCP with p53 tetrameric domain to improve its affinity. FLSs were isolated and cultured from rheumatoid arthritis (RA) patients and control subjects. A co-culture system of peripheral mononuclear cells (PBMCs) and FLSs was used. FLSs proliferation, migration, and invasion were measured by MTT, scratch test, and Transwell chamber. Supernatants were measured for cytokines, chemokines, metalloproteinases, and anti-CCP antibodies by Luminex liquid phase protein chip and ELISA. TeAb-CCP significantly inhibited FLSs proliferation in a dose-dependent mode, with maximal action at concentration of 100 μg/ml on the 7th day in the co-culture system with PBMCs and FLSs, but not the same with only FLSs. TeAb-CCP significantly suppressed FLSs migration and invasive ability compared with the controls. Significantly lower levels of interleukin (IL)-6, IL-8, RANKL, protein arginine deiminase (PAD)-2, PAD4, metalloproteinase (MMP)-1 and MMP-3 and anti-CCP antibodies were found in co-culture supernatant of TeAb-CCP group. In contrast, transforming growth factor-β (TGF-β) and tissue inhibitor of metalloproteinases-2 (TIMP-2) was significantly increased in the TeAb-CCP group. No significant difference of IL-1a, IL-10, IL-17, TNFα, VEGF, and FGF was found between two groups. As a blocking antibody, TeAb-CCP can significantly inhibit PBMCs of RA to produce pro-inflammatory mediators, and furthermore, inhibit the proliferation, activation, migration, and invasion of FLSs in vitro. In turn, it is suggested that citrullinated modified self-epitopes may be a new target for RA therapy.






Similar content being viewed by others
References
Muller, S., and M. Radic. 2015. Citrullinated autoantigens: From diagnostic markers to pathogenetic mechanisms. Clinical Reviews in Allergy and Immunology 49: 232–239.
Olsen, I., S.K. Singhrao, and J. Potempa. 2018. Citrullination as a plausible link to periodontitis, rheumatoid arthritis, atherosclerosis and Alzheimer's disease. Journal of Oral Microbiology 10: 1487742.
Aletaha, D., T. Neogi, A.J. Silman, et al. 2010. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis and Rheumatism 62: 2569–2581.
Ioan-Facsinay, A., H. El-Bannoudi, Scherer Hu, et al. 2011. Anti-cyclic citrullinated peptide antibodies are a collection of anti-citrullinated protein antibodies and contain overlapping and non-overlapping reactivities. Annals of the Rheumatic Diseases 70: 188–193.
Hongbin, Li, Tie Ning, Jia Yong-Feng, et al. 2012. Association of synovial cyclic citrullinated peptide expression with Th17/Treg imbalance and synovitis in rheumatoid arthritis patients.16 (4): 224–228.
van Venrooij, W.J., J.J. van Beers, and G.J. Pruijn. 2011. Anti-CCP antibodies: The past, the present and the future. Nature Reviews Rheumatology 7: 391–398.
Darrah, E., and F. Andrade. 2018. Rheumatoid arthritis and citrullination. Current Opinion in Rheumatology 30: 72–78.
Deane, K.D. 2018. Preclinical rheumatoid arthritis and rheumatoid arthritis prevention. Current Rheumatology Reports 20: 50.
Alunno, A., O. Bistoni, F. Pratesi, et al. 2018. Anti-citrullinated alpha enolase antibodies, interstitial lung disease and bone erosion in rheumatoid arthritis. Rheumatology (Oxford) 57: 850–855.
Chen, J., S. Song, Y. Liu, et al. 2018. Autoreactive T cells to citrullinated HSP90 are associated with interstitial lung disease in rheumatoid arthritis. International Journal of Rheumatic Diseases 21: 1398–1405.
Mok, C.C. 2013. Rituximab for the treatment of rheumatoid arthritis: An update. Drug Design, Development and Therapy 8: 87–100.
Dong, X., Z. Zheng, Y. Zhai, et al. 2018. ACPA mediates the interplay between innate and adaptive immunity in rheumatoid arthritis. Autoimmunity Reviews 17: 845–853.
Rims, C., H. Uchtenhagen, M.J. Kaplan, et al. 2019. Citrullinated Aggrecan epitopes as targets of autoreactive CD4+ T cells in patients with rheumatoid arthritis. Arthritis & Rhematology 71: 518–528.
Rönnelid, J., M. Hansson, L. Mathsson-Alm, et al. 2018. Anticitrullinated protein/peptide antibody multiplexing defines an extended group of ACPA-positive rheumatoid arthritis patients with distinct genetic and environmental determinants. Annals of the Rheumatic Diseases 77: 203–211.
Hill, J.A., S. Southwood, A. Sette, A.M. Jevnikar, D.A. Bell, and E. Cairns. 2003. Cutting edge: The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA-DRB1*0401 MHC class II molecule. Journal of Immunology 171: 538–541.
Margulies, D.H. 2018. How MHC molecules grab citrullinated peptides to foster rheumatoid arthritis. The Journal of Biological Chemistry 293: 3252–3253.
Hill, J.A., D.A. Bell, W. Brintnell, et al. 2008. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. The Journal of Experimental Medicine 205: 967–979.
van der Woude, D., S. Rantapää-Dahlqvist, A. Ioan-Facsinay, et al. 2010. Epitope spreading of the anti-citrullinated protein antibody response occurs before disease onset and is associated with the disease course of early arthritis. Annals of the Rheumatic Diseases 69: 1554–1561.
de Moel, E.C., V. Derksen, G. Stoeken, et al. 2018. Baseline autoantibody profile in rheumatoid arthritis is associated with early treatment response but not long-term outcomes. Arthritis Research & Therapy 20 (1): 33.
Ge, C., B. Xu, B. Liang, E. Lönnblom, S.L. Lundström, R.A. Zubarev, B. Ayoglu, P. Nilsson, T. Skogh, A. Kastbom, V. Malmström, L. Klareskog, R.E.M. Toes, T. Rispens, D. Dobritzsch, and R. Holmdahl. 2019. Structural basis of cross-reactivity of anti-Citrullinated protein antibodies. Arthritis & Rhematology 71 (2): 210–221.
Elliott, S.E., S. Kongpachith, N. Lingampalli, J.Z. Adamska, B.J. Cannon, R. Mao, L.K. Blum, and W.H. Robinson. 2018. Affinity maturation drives epitope spreading and generation of proinflammatory anti-Citrullinated protein antibodies in rheumatoid arthritis. Arthritis & Rhematology 70 (12): 1946–1958.
Trier, N.H., and G. Houen. 2017. Epitope Specificity of Anti-Citrullinated Protein Antibodies. Antibodies (Basel) 6 (1).
Willemze, A., S. Böhringer, R. Knevel, et al. 2012. The ACPA recognition profile and subgrouping of ACPA-positive RA patients. Annals of the Rheumatic Diseases 71: 268–274.
Sokolove, J., R. Bromberg, K.D. Deane, et al. 2012. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One 7: e35296.
Sun, M., B. Rethi, A. Krishnamurthy, V. Joshua, A. Circiumaru, A.H. Hensvold, E. Ossipova, C. Grönwall, Y. Liu, M. Engstrom, S.B. Catrina, J. Steen, V. Malmstrom, L. Klareskog, C. Svensson, C. Ospelt, H. Wähämaa, and A.I. Catrina. 2019. Anticitrullinated protein antibodies facilitate migration of synovial tissue-derived fibroblasts. Annals of the Rheumatic Diseases 78 (12): 1621–1631.
Bustamante, M.F., R. Garcia-Carbonell, K.D. Whisenant, and M. Guma. 2017. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Research & Therapy 19 (1): 110.
Ganesan, R., and M. Rasool. 2017. Fibroblast-like synoviocytes-dependent effector molecules as a critical mediator for rheumatoid arthritis: Current status and future directions. International Reviews of Immunology 36 (1): 20–30.
Hu, X.X., Y.J. Wu, J. Zhang, and W. Wei. 2019. T-cells interact with B cells, dendritic cells, and fibroblast-like synoviocytes as hub-like key cells in rheumatoid arthritis. International Immunopharmacology 70: 428–434.
Ahmad, Z.A., S.K. Yeap, A.M. Ali, W.Y. Ho, N.B. Alitheen, and M. Hamid. 2012. scFv antibody: principles and clinical application. Clinical & Developmental Immunology 2012: 980250.
Fan, L.Y., He Dy, Q. Wang, et al. 2012. Citrullinated vimentin stimulates proliferation, pro-inflammatory cytokine secretion, and PADI4 and RANKL expression of fibroblast-like synoviocytes in rheumatoid arthritis. Scandinavian Journal of Rheumatology 41: 354–358.
Fan, L., Q. Wang, R. Liu, M. Zong, D. He, H. Zhang, Y. Ding, and J. Ma. 2012. Citrullinated fibronectin inhibits apoptosis and promotes the secretion of pro-inflammatory cytokines in fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Research & Therapy 14 (6): R266.
Hensvold, A.H., V. Joshua, W. Li, et al. 2015. Serum RANKL levels associate with anti- citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Research & Therapy 17: 239.
Chang, X., R. Yamada, A. Suzuki, et al. 2005. Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford) 44: 40–50.
Tang, Y., B. Wang, X. Sun, H. Li, X. Ouyang, J. Wei, B. Dai, Y. Zhang, and X. Li. 2017. Rheumatoid arthritis fibroblast-like synoviocytes co-cultured with PBMC increased peripheral CD4+ CXCR5+ ICOS+ T cell numbers. Clinical and Experimental Immunology 190 (3): 384–393.
Funding
This work was funded by grants from the Natural Science Foundation of China (81360460 and 81660276) and Inner Mongolia grassland talents fund.
Author information
Authors and Affiliations
Contributions
WJ, XXK, and HBL had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study design: XXK and HBL. Acquisition of data and analysis and interpretation of data: WJ, TN, XXK, and HBL. Manuscript preparation: WJ and HBL. Statistical analysis: WJ. All original data used to support the findings of this study are included within the article. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Tie, N., Li, H. et al. Inhibitory Effect of Tetramerized Single-Chain Variable Fragment of Anti-Cyclic Citrullinated Peptide Antibodies on the Proliferation, Activation, and Secretion of Cytokines of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis In Vitro Co-Culture System. Inflammation 43, 2245–2255 (2020). https://doi.org/10.1007/s10753-020-01292-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01292-z