Abstract
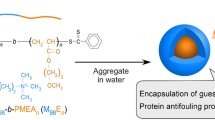
Polymer micelles with a tunable drug release would be suitable for the concept of drug delivery system. We constructed photo-responsive polymer micelles from amphiphilic block copolymers. The polymer micelles were synthesized by mechanochemical solid-state copolymerization of poly[N-(2-hydroxypropyl)methacrylamide] (PHPMA) and 4,5-dimethoxy-2-nitrobenzyl methacrylate as a photosensitive moiety. The above mechanochemical solid-state copolymerization was performed by vibratory-ball milling at 30 Hz in a nitrogen atmosphere with the use of an agate vessel and an agate ball to yield amphiphilic block copolymers (PHPMA-b-PDNMA). Spherical polymer micelles were formed by the self-assembly of PHPMA-b-PDNMA. The diameter of the PHPMA-b-PDNMA micelles was in the range of 130-200 nm. The PHPMA-b-PDNMA micelles loaded with the antitumor drug 5-fluorouracil (5-FU) showed photo irradiation induced time-dependent release of 5-FU with an associated decrease of micellar size. The drug release profile of the PHPMA-b-PDNMA micelles followed a clear sigmoid curve. Our approach provides a controlled drug release system through the use of photo-responsive polymer micelles, accompanied by the gradual decrease of micellar size.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Li KC, Pandit SD, Guccione S, Bednarski MD. Molecular imaging applications in nanomedicine. Biomed Microdevices. 2004;6:113–6.
Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–30.
Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, Guthi JS, et al. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–30.
Skinner SA, Tutton PJM, O’Brien PE. Microvascular architecture of experimental colon tumors in the rat. Cancer Res. 1990;50:2411–7.
Suzuki M, Hori K, Abe I, Saito S, Sato H. A new approach to cancer chemotherapy: selective enhancement of tumor blood flow with angiotensin II. J Natl Cancer Inst. 1981;67:663–9.
Maeda H, Matsumura Y. Tumoritropic and lymphotropic principles of macromolecular drugs. Crit Rev Ther Drug Carr Syst. 1989;6:193–210.
Iwai K, Maeda H, Konno T. Use of oily contrast medium for selective drug targeting to tumor: enhanced therapeutic effect and X-ray image. Cancer Res. 1984;44:2115–21.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Cammasa S, Suzuki K, Sone C, Sakurai Y, Kataoka K, Okano T. Thermo-responsive polymer nanoparticles with a core-shell micelle structure as site-specific drug carriers. J Controlled Release. 1997;48:157–64.
Chung JE, Yokoyama M, Okano T. Inner core segment design for drug delivery control of thermo-responsive polymeric micelles. J Controlled Release. 2000;65:93–103.
Liang X, Liu F, Kozlovskaya V, Palchak Z, Kharlampieva E. Thermoresponsive micelles from double LCST-Poly(3-methyl-N-vinylcaprolactam) block copolymers for cancer therapy. ACS Macro Lett. 2015;4:308–11.
Hassanzadeh F, Farzan M, Varshosaz J, Khodarahmi GA, Maaleki S, Rostami M. Poly (ethylene-co-vinyl alcohol)-based polymeric thermo-responsive nanocarriers for controlled delivery of epirubicin to hepatocellular carcinoma. Res Pharm Sci. 2017;12:107–18.
Zhao Y. Photocontrollable block copolymer micelles: what can we control? J Mater Chem. 2009;19:4887–95.
Ercole F, Davis TP, Evans RA. Photo-responsive systems and biomaterials: photochromic polymers, light-triggered self-assembly, surface modification, fluorescence modulation and beyond. Polym Chem. 2010;1:37–54.
Schumers JM, Fustinand CA, Gohy JF. Light-responsive block copolymers. Macromol Rapid Commun. 2010;31:1588–607.
Pasparakis G, Manouras T, Argitis P, Vamvakaki M. Photodegradable polymers for biotechnological applications. Macromol Rapid Commun. 2012;33:183–98.
Zhao Y. Light-responsive block copolymer micelles. Macromolecules. 2012;45:3647–57.
Husseini GA, Myrup GD, Pitt WG, Christensen DA, Rapoport NY. Factors affecting acoustically triggered release of drugs from polymeric micelles. J Control Release. 2000;69:43–52.
Gao Z, Fain HD, Rapoport N. Ultrasound-enhanced tumor targeting of polymeric micellar drug carriers. Mol Pharm. 2004;1:317–30.
Zhang H, Xia H, Wang J, Li Y. High intensity focused ultrasound-responsive release behavior of PLA-b-PEG copolymer micelles. J Control Release. 2009;139:31–9.
Wu P, Jia Y, Qu F, Sun Y, Wang P, Zhang K, et al. Ultrasound-responsive polymeric micelles for sonoporation-assisted site-specific therapeutic action. ACS Appl Mater Interfaces. 2017;9:25706–16.
Bae Y, Nishiyama N, Fukushima S, Koyama H, Matsumura Y, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability, controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjugate Chem. 2005;16:122–30.
Convertine AJ, Diab C, Prieve M, Paschal A, Hoffman AS, Johnson PH, et al. pH-responsive polymeric micelle carriers for siRNA drugs. Biomacromolecules. 2010;11:2904–11.
Kondo S, Yamamoto K, Sawama Y, Sasai Y, Yamauchi Y, Kuzuya M. Characterization of novel pH-sensitive polymeric micelles prepared by the self-assembly of amphiphilic block copolymer with poly-4-vinylpyridine block synthesized by mechanochemical solid-state polymerization. Chem Pharm Bull. 2011;59:1200–2.
Kondo S, Asano Y, Koizumi N, Tatematsu K, Sawama Y, Sasai Y, et al. Novel pH-responsive polymeric micelles prepared through self-assembly of amphiphilic block copolymer with poly-4-vinylpyridine block synthesized by mechanochemical solid-state polymerization. Chem Pharm Bull. 2015;63:489–94.
Hiruta Y, Kanda Y, Katsuyama N, Kanazawa H. Dual temperature- and pH-responsive polymeric micelle for selective and efficient two-step doxorubicin delivery. RSC Adv. 2017;7:29540–49.
Li Q, Yao W, Yu X, Zhang B, Dong J, Jin Y. Drug-loaded pH-responsive polymeric micelles: simulations and experiments of micelle formation, drug loading and drug release. Colloids Surf B Biointerfaces. 2017;158:709–16.
Zhao H, Sterner ES, Coughlin EB, Theato P. o-nitrobenzyl alcohol derivatives: opportunities in polymer and materials science. Macromolecules. 2012;45:1723–36.
Zhao Y. Rational design of light-controllable polymer micelles. Chem Rec. 2007;7:286–94.
Gohy J-F, Zhao Y. Photo-responsive block copolymer micelles: design and behavior. Chem Soc Rev. 2013;42:7117–29.
Abreu CMR, Mendonça PV, Serra AC, Coelho JFJ, Popov AV, Guliashvili T. Accelerated ambient-temperature ATRP of methyl acrylate in alcohol-water solutions with a mixed transition-metal catalyst system. Macromol Chem Phys. 2012;213:1677–87.
Ding M, Jiang X, Zhang L, Cheng Z, Zhu X. Recent progress on transition metal catalyst separation and recycling in ATRP. Macromol Rapid Commun. 2015;36:1702–21.
Frazer L. Radical departure: polymerization does more with less. Environ Health Perspect. 2007;115:A258–61.
Kuzuya M, Kondo S, Noguchi A. A new development of mechanochemical solid-state polymerization of vinyl monomers: prodrug syntheses and its detailed mechanistic study. Macromolecules. 1991;24:4047–53.
Kuzuya M, Kondo S, Noguchi A, Noda N. Mechanistic study on mechanochemical polymerization of acrylamide. J Polym Sci Part A Polym Chem. 1991;29:489–94.
Kuzuya M, Kondo S, Noguchi A, Noda N. Nature of mechanoradical formation and reactivity with oxygen in methacrylic vinyl polymers. J Polym Sci Part B Polym Phys. 1992;30:97–103.
Kondo S, Sasai Y, Hosaka S, Ishikawa T, Kuzuya M. Kinetic analysis of the mechanolysis of polymethylmethacrylate in the course of vibratory ball milling at various mechanical energy. J Polym Sci Part A Polym Chem. 2004;42:4161–67.
Kuzuya M, Yamauchi Y, Kondo S. Mechanolysis of glucose-based polysaccharides as studied by electron spin resonance. J Phys Chem B. 1999;103:8051–9.
Sasai Y, Yamauchi Y, Kondo S, Kuzuya M. Nature of mechanoradical formation of substituted celluloses as studied by electron spin resonance. Chem Pharm Bull. 2004;52:339–44.
Doi N, Sasai Y, Yamauchi Y, Adachi T, Kuzuya M, Kondo S. Kinetic analysis of mechanoradical formation during the mechanolysis of dextran and glycogen. Beilstein J Org Chem. 2017;13:1174–83.
Kondo S, Hatakeyama I, Hosaka S, Kuzuya M. Mechanochemical solid-state polymerization (X): the influence of copolymer structure in copolymeric prodrugs on the nature of drug release. Chem Pharm Bull. 2000;48:1882–5.
Kondo S, Mori H, Sasai Y, Kuzuya M. Conventional synthesis of amphiphilic block copolymer utilized for polymeric micelle by mechanochemical solid-state polymerization. Chem Pharm Bull. 2007;55:389–92.
Doi N, Sasai Y, Yamauchi Y, Adachi T, Kuzuya M, Kondo S. Development of novel polymeric prodrugs synthesized by mechanochemical solid-state copolymerization of hydroxyethylcellulose and vinyl monomers. Chem Pharm Bull. 2015;63:992–7.
Doi N, Sasai Y, Yamauchi Y, Adachi T, Kuzuya M, Kondo S. A novel polymeric prodrugs synthesized by mechanochemical solid-state copolymerization of glucose-based polysaccharides and vinyl monomers. Int J Pharm Sci Invent. 2017;6:38–46.
Duncan R. The drawing era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–60.
Kopeček J, Kopečková P, Minko T, Lu Z. HPMA copolymer-anticancer drug conjugates: design, activity, and mechanism of action. Eur J Pharm Biopharm. 2000;50:61–81.
Říhová B, Kubáčková K. Clinical implications of N-(2-hydroxypropyl)methacrylamide copolymers. Curr Pharm Biotechnol. 2003;4:311–22.
Talelli M, Rijcken CJF, van Nostrum CF, Storm G, Hennink WE. Micelles based on HPMA copolymers. Adv Drug Deliv Rev. 2010;62:231–9.
Kim K, Kwon S, Park JH, Chung H, Jeong SY, Kwon IC, et al. Physicochemical characterizations of self-assembled nanoparticles of glycol chitosan-deoxycholic acid conjugates. Biomacromolecules. 2005;6:1154–8.
Nam HY, Kwon SM, Chung H, Lee SY, Kwon SH, Jeon H, et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J Control Release. 2009;135:259–67.
Stankovich S, Piner RD, Nguyen S-BT, Ruoff RS. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon. 2006;44:3342–47.
Park C, Lee IH, Lee S, Song Y, Rhue M, Kim C. Cyclodextrin-covered organic nanotubes derived from self-assembly of dendrons and their supramolecular transformation. Proc Natl Acad Sci USA. 2006;103:1199–203.
Li X, Mya KY, Ni X, He C, Leong KW, Li J. Dynamic and static light scattering studies on self-aggregation behavior of biodegradable amphiphilic poly(ethylene oxide)-poly[(R)-3-hydroxybutyrate]-poly(ethylene oxide) triblock copolymers in aqueous solution. J Phys Chem B. 2006;110:5920–6.
Acknowledgements
This work was supported by a grant from the OGAWA Science and Technology Foundation. We also give thanks to Springer Nature Author Services for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Doi, N., Yamauchi, Y., Ikegami, R. et al. Photo-responsive polymer micelles from o-nitrobenzyl ester-based amphiphilic block copolymers synthesized by mechanochemical solid-state copolymerization. Polym J 52, 1375–1385 (2020). https://doi.org/10.1038/s41428-020-0387-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0387-9
This article is cited by
-
Recent progress in photoreactive crosslinkers in polymer network materials toward advanced photocontrollability
Polymer Journal (2024)
-
Enzyme-catalyzed propagation of cello-oligosaccharide chains from bifunctional oligomeric primers for the preparation of block co-oligomers and their crystalline assemblies
Polymer Journal (2021)