Histological, Physiological and Transcriptomic Analysis Reveal Gibberellin-Induced Axillary Meristem Formation in Garlic (Allium sativum)
Abstract
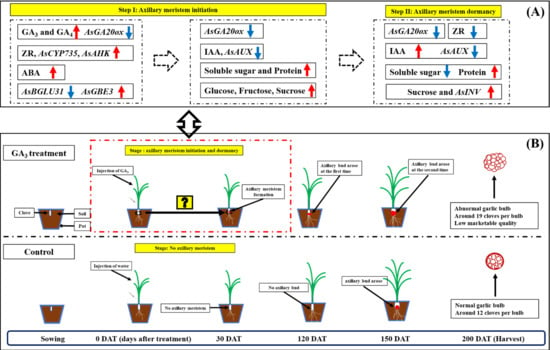
:1. Introduction
2. Results
2.1. Injection of GA3 Promotes Axillary Meristem Formation of Garlic
2.2. Impact of Injection of GA3 on Plant Hormone Level in Garlic Stems
2.3. Effect of GA3 Treatment on Sugars and Soluble Protein Content in Garlic Stems
2.4. Transcriptome Analysis
2.5. Dynamic Expression of AsGA20ox, AsAUX, AsCYP735, AsAHK, AsBGLU31 and AsINV
2.6. Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Materials, GA3 Treatment and Growth Conditions
4.2. Histology of Axillary Meristem and Axillary Bud
4.3. Assessment of Endogenous Plant Hormone Levels
4.4. Evaluation of Sugars Content and Soluble Protein Content
4.5. Transcriptome Analysis
4.6. Quantitative Real-Time PCR (qRT-PCR) Validation of Gene Expression Levels
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Li, J. Molecular basis of plant architecture. Annu. Rev. Plant Biol. 2008, 59, 253–279. [Google Scholar] [CrossRef] [PubMed]
- Domagalska, M.A.; Leyser, O. Signal integration in the control of shoot branching. Nat. Rev. Mol. Cell Biol. 2011, 12, 211–221. [Google Scholar] [CrossRef]
- Janssen, B.J.; Drummond, R.S.M.; Snowden, K.C. Regulation of axillary shoot development. Curr. Opin. Plant Biol. 2014, 17, 28–35. [Google Scholar] [CrossRef]
- Barbier, F.; Perez-Garcia, M.-D.; Barrière, Q.; Sakr, S.; Lecerf, M.; Péron, T.; Bertheloot, J.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef] [Green Version]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An update on the signals controlling shoot branching. Trends Plant Sci. 2019, 24, 220–236. [Google Scholar] [CrossRef] [PubMed]
- Evers, J.B.; van der Krol, A.R.; Vos, J.; Struik, P.C. Understanding shoot branching by modelling form and function. Trends Plant Sci. 2011, 16, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.F.; Lunn, J.E.; Beveridge, C.A. Ready, steady, go! A sugar hit starts the race to shoot branching. Curr. Opin. Plant Biol. 2015, 25, 39–45. [Google Scholar] [CrossRef]
- Chen, X.-J.; Xia, X.-J.; Guo, X.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Yu, J.-Q. Apoplastic H2O2 plays a critical role in axillary bud outgrowth by altering auxin and cytokinin homeostasis in tomato plants. New Phytol. 2016, 211, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.; Chen, D.; Liu, G.; Liang, Y.; Huang, L.; Meng, X.; Chu, J.; Sun, X.; Dong, G.; Yuan, Y.; et al. Strigolactones regulate rice tiller angle by attenuating shoot gravitropism through inhibiting auxin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 11199–11204. [Google Scholar] [CrossRef] [Green Version]
- Hussien, A.; Tavakol, E.; Horner, D.S.; Muñoz-Amatriaín, M.; Muehlbauer, G.J.; Rossini, L. Genetics of tillering in rice and barley. Plant Genome 2014, 7, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Li, G.; Chen, X.; Xing, L.; Ma, J.; Zhang, D.; Ge, H.; Han, M.; Sha, G.; An, N. Role of cytokinin, strigolactone, and auxin export on outgrowth of axillary buds in apple. Front. Plant Sci. 2019, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Bredmose, N.; Kristiansen, K.; Nørbæk, R.; Christensen, L.P.; Hansen-Møller, J. Changes in concentrations of cytokinins (CKs) in root and axillary bud tissue of miniature rose suggest that local CK biosynthesis and zeatin-type CKs play important roles in axillary bud growth. J. Plant Growth Regul. 2005, 24, 238–250. [Google Scholar] [CrossRef]
- Kamenetsky, R. Garlic: Botany and horticulture. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; Volume 33, pp. 123–172. [Google Scholar]
- Etoh, T.; Simon, P.W. Diversity, fertility and seed production of garlic. In Allium Crop Science: Recent Advances; CABI Publishing: Wallingford, UK, 2002. [Google Scholar]
- Achard, P.; Genschik, P. Releasing the brakes of plant growth: How GAs shutdown DELLA proteins. J. Exp. Bot. 2009, 60, 1085–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapelli, S.; Kinet, J.M. Plant-growth regulator and graft control of axillary bud formation and development in the to-2 mutant tomato. Plant Growth Regul. 1992, 11, 385–390. [Google Scholar] [CrossRef]
- Elfving, D.C.; Visser, D.B.; Henry, J.L. Gibberellins stimulate lateral branch development in young sweet cherry trees in the orchard. Int. J. Fruit Sci. 2011, 11, 41–54. [Google Scholar] [CrossRef]
- Ni, J.; Gao, C.C.; Chen, M.S.; Pan, B.Z.; Ye, K.Q.; Xu, Z.F. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, H.; Shiraiwa, N.; Itai, A.; Honda, I. Involvement of gibberellins in the regulation of tillering in Welsh onion (Allium fistulosum L.). Horticult. J. 2015, 84, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Deng, R.; Huang, C.; Cheng, Z.; Meng, H. Exogenous gibberellins alter morphology and nutritional traits of garlic (Allium sativum L.) bulb. Sci. Hortic. 2019, 246, 298–306. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Yang, F.; Qi, X.; Ahmad, H.; Wu, C.; Cheng, Z. Effect of the mode and time of gibberellic acid treatment on plant architecture and bulb structure in garlic (Allium sativum L.). Sci. Hortic. 2019, 257, 108723. [Google Scholar] [CrossRef]
- Grbić, V.; Bleecker, A.B. Axillary meristem development in Arabidopsis thaliana. Plant J. 2000, 21, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, J. Branching in rice. Curr. Opin. Plant Biol. 2011, 14, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A.; Weller, J.L.; Singer, S.R.; Hofer, J.M.I. Axillary meristem development. budding relationships between networks controlling flowering, branching and photoperiod responsiveness. Plant Physiol. 2003, 131, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohkin Shalom, S.; Gillett, D.; Zemach, H.; Kimhi, S.; Forer, I.; Zutahy, Y.; Tam, Y.; Teper-Bamnolker, P.; Kamenetsky, R.; Eshel, D. Storage temperature controls the timing of garlic bulb formation via shoot apical meristem termination. Planta 2015, 242, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.P.; Resende, F.V.; Zucchi, M.I.; Pinheiro, J.B. SSR-based genetic diversity and structure of garlic accessions from Brazil. Genetica 2014, 142, 419–431. [Google Scholar] [CrossRef]
- Kumar, M.; Rakesh Sharma, V.; Kumar, V.; Sirohi, U.; Chaudhary, V.; Sharma, S.; Saripalli, G.; Naresh, R.K.; Yadav, H.K.; Sharma, S. Genetic diversity and population structure analysis of Indian garlic (Allium sativum L.) collection using SSR markers. Physiol. Mol. Biol. Plants 2019, 25, 377–386. [Google Scholar] [CrossRef]
- Zhao, W.-G.; Chung, J.-W.; Lee, G.-A.; Ma, K.-H.; Kim, H.-H.; Kim, K.-T.; Chung, I.-M.; Lee, J.-K.; Kim, N.-S.; Kim, S.-M.; et al. Molecular genetic diversity and population structure of a selected core set in garlic and its relatives using novel SSR markers. Plant Breed. 2011, 130, 46–54. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Shalom, S.R.; Faigenboim-Doron, A.; Teper-Bamnolker, P.; Salam, B.B.; Daus, A.; Kamenetsky, R.; Eshel, D. Differential carbohydrate gene expression during preplanting temperature treatments controls meristem termination and bulbing in garlic. Environ. Exp. Bot. 2018, 150, 280–291. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, S.; Meng, F.; Liu, S. De novo assembly and characterization of the garlic (Allium sativum) bud transcriptome by Illumina sequencing. Plant Cell Rep. 2012, 31, 1823–1828. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Faigenboim, A.; Mayer, E.S.; Michael, T.B.; Gershberg, C.; Kimhi, S.; Esquira, I.; Shalom, S.R.; Eshel, D.; Rabinowitch, H.D.; et al. Integrated transcriptome catalogue and organ-specific profiling of gene expression in fertile garlic (Allium sativum L.). BMC Genomics 2015, 16, 12. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Fambrini, M.; Mariotti, L.; Parlanti, S.; Picciarelli, P.; Salvini, M.; Ceccarelli, N.; Pugliesi, C. The extreme dwarf phenotype of the GA-sensitive mutant of sunflower, dwarf2, is generated by a deletion in the ent-kaurenoic acid oxidase1 (HaKAO1) gene sequence. Plant Mol.Biol. 2011, 75, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Que, F.; Xu, Z.S.; Wang, F.; Xiong, A.S. Exogenous gibberellin enhances secondary xylem development and lignification in carrot taproot. Protoplasma 2017, 254, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.; Picciarelli, P.; Lombardi, L.; Ceccarelli, N. Fruit-set and early fruit growth in tomato are associated with increases in indoleacetic acid, cytokinin, and bioactive gibberellin contents. J. Plant Growth Regul. 2011, 30, 405. [Google Scholar] [CrossRef]
- Rizza, A.; Jones, A.M. The makings of a gradient: Spatiotemporal distribution of gibberellins in plant development. Curr. Opin. Plant Biol. 2019, 47, 9–15. [Google Scholar] [CrossRef]
- Huang, S.; Raman, A.S.; Ream, J.E.; Fujiwara, H.; Cerny, R.E.; Brown, S.M. Overexpression of 20-oxidase confers a gibberellin-overproduction phenotype in Arabidopsis. Plant Physiol. 1998, 118, 773–781. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Yao, Y.; Ni, Z.; Sun, Q. Cloning and characterization of an up-regulated GA 20-oxidase gene in hybrid maize. Prog. Nat. Sci. 2009, 19, 161–166. [Google Scholar] [CrossRef]
- Jeon, H.W.; Cho, J.S.; Park, E.J.; Han, K.H.; Choi, Y.I.; Ko, J.H. Developing xylem-preferential expression of PdGA20ox1, a gibberellin 20-oxidase 1 from Pinus densiflora, improves woody biomass production in a hybrid poplar. Plant Biotechnol. J. 2016, 14, 1161–1170. [Google Scholar] [CrossRef]
- Toyomasu, T.; Kawaide, H.; Sekimoto, H.; von Numers, C.; Phillips, A.L.; Hedden, P.; Kamiya, Y. Cloning and characterization of a cDNA encoding gibberellin 20-oxidase from rice (Oryza sativa) seedlings. Physiologia Plantarum 1997, 99, 111–118. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Wang, J.-G.; Wang, L.-Y.; Wang, J.-F.; Wang, Q.; Yu, P.; Bai, M.-Y.; Fan, M. Gibberellin repression of axillary bud formation in Arabidopsis by modulation of DELLA-SPL9 complex activity. J. Integr. Plant Biol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-J.; Huang, C.-P.; Tong, P.-J.; Yang, X.; Cui, M.-M.; Cheng, Z.-H. Response of axillary bud development in garlic (Allium sativum L.) to seed cloves soaked in gibberellic acid (GA3) solution. J. Integr. Agric. 2020, 19, 1044–1054. [Google Scholar] [CrossRef]
- Koorneef, M.; Elgersma, A.; Hanhart, C.J.; van Loenen-Martinet, E.P.; van Rijn, L.; Zeevaart, J.A.D. A gibberellin insensitive mutant of Arabidopsis thaliana. Physiologia Plantarum 1985, 65, 33–39. [Google Scholar] [CrossRef]
- Zawaski, C.; Busov, V.B. Roles of gibberellin catabolism and signaling in growth and physiological response to drought and short-day photoperiods in Populus trees. PLoS ONE 2014, 9, e86217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, T.K.; Case, D.B.; Jacobs, W.P. Auxin-gibberellin interaction in apical dominance. Plant Physiol. 1967, 42, 1329–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Martínez, J.A.; Poza-Carrión, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef]
- Mauriat, M.; Sandberg, L.G.; Moritz, T. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. Plant J. 2011, 67, 805–816. [Google Scholar] [CrossRef]
- Liao, Z.; Yu, H.; Duan, J.; Yuan, K.; Yu, C.; Meng, X.; Kou, L.; Chen, M.; Jing, Y.; Liu, G.; et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat. Commun. 2019, 10, 2738. [Google Scholar] [CrossRef] [Green Version]
- Band, L.R.; Preston, S.P. Parameter inference to motivate asymptotic model reduction: An analysis of the gibberellin biosynthesis pathway. J. Theor. Biol. 2018, 457, 66–78. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, J.; Shi, B.; Yu, T.; Qi, J.; Meyerowitz, E.M.; Jiao, Y. The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell 2014, 26, 2055–2067. [Google Scholar] [CrossRef] [Green Version]
- Emery, R.J.N.; Longnecker, N.E.; Atkins, C.A. Branch development in Lupinus angustifolius L. II. Relationship with endogenous ABA, IAA and cytokinins in axillary and main stem buds. J. Exp. Bot. 1998, 49, 555–562. [Google Scholar]
- Zhuang, L.; Ge, Y.; Wang, J.; Yu, J.; Yang, Z.; Huang, B. Gibberellic acid inhibition of tillering in tall fescue involving crosstalks with cytokinins and transcriptional regulation of genes controlling axillary bud outgrowth. Plant Sci. 2019, 287, 110168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiao, Y.L. Axillary meristem initiation—A way to branch out. Curr. Opin. Plant Biol. 2018, 41, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, J.; Dong, X.; Cai, Z.; Tian, W.; Wang, X. Short-term and continuing stresses differentially interplay with multiple hormones to regulate plant survival and growth. Mol. Plant 2014, 7, 841–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Kohlen, W.; Rossmann, S.; Vernoux, T.; Theres, K. Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. Plant Cell 2014, 26, 2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pautler, M.; Tanaka, W.; Hirano, H.-Y.; Jackson, D. Grass meristems I: Shoot apical meristem maintenance, axillary meristem determinacy and the floral transition. Plant Cell Physiol. 2013, 54, 302–312. [Google Scholar] [CrossRef]
- Patrick, J.W.; Botha, F.C.; Birch, R.G. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar] [CrossRef]
- Bonhomme, M.; Peuch, M.; Ameglio, T.; Rageau, R.; Guilliot, A.; Decourteix, M.; Alves, G.; Sakr, S.; Lacointe, A. Carbohydrate uptake from xylem vessels and its distribution among stem tissues and buds in walnut (Juglans regia L.). Tree Physiol. 2009, 30, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Zhang, M.; Wu, Y.; Gao, X.; Li, X.; Wang, W. The roles of auxin in regulating “shoot branching” of Cremastra appendiculata. J. Plant Growth Regul. 2017, 36, 281–289. [Google Scholar] [CrossRef]
- Rabot, A.; Henry, C.; Ben Baaziz, K.; Mortreau, E.; Azri, W.; Lothier, J.; Hamama, L.; Boummaza, R.; Leduc, N.; Pelleschi-Travier, S.; et al. Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol. 2012, 53, 1068–1082. [Google Scholar] [CrossRef] [Green Version]
- Singhania, R.R.; Patel, A.K.; Pandey, A.; Ganansounou, E. Genetic modification: A tool for enhancing beta-glucosidase production for biofuel application. Bioresource Technol. 2017, 245, 1352–1361. [Google Scholar] [CrossRef]
- Otori, K.; Tamoi, M.; Tanabe, N.; Shigeoka, S. Enhancements in sucrose biosynthesis capacity affect shoot branching in Arabidopsis. Biosci. Biotechnol. Biochem. 2017, 81, 1470–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salam, B.B.; Malka, S.K.; Zhu, X.; Gong, H.; Ziv, C.; Teper-Bamnolker, P.; Ori, N.; Jiang, J.; Eshel, D. Etiolated stem branching Is a result of systemic signaling associated with sucrose level. Plant Physiol. 2017, 175, 734–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquat, C.; Vandamme, M.; Gendraud, M.; Pétel, G. Dormancy in vegetative buds of peach: Relation between carbohydrate absorption potentials and carbohydrate concentration in the bud during dormancy and its release. Sci. Hortic. 1999, 79, 151–162. [Google Scholar] [CrossRef]
- Richardson, A.C.; Walton, E.F.; Meekings, J.S.; Boldingh, H.L. Carbohydrate changes in kiwifruit buds during the onset and release from dormancy. Sci. Hortic. 2010, 124, 463–468. [Google Scholar] [CrossRef]
- Sherson, S.M.; Alford, H.L.; Forbes, S.M.; Wallace, G.; Smith, S.M. Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J. Exp. Bot. 2003, 54, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Ni, D.-A.; Ruan, Y.-L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef] [Green Version]
- Heyer, A.G.; Raap, M.; Schroeer, B.; Marty, B.; Willmitzer, L. Cell wall invertase expression at the apical meristem alters floral, architectural, and reproductive traits in Arabidopsis thaliana. Plant J. 2004, 39, 161–169. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Wang, W. Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol. 2001, 127, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Li, G.; Yi, G.-X.; Wang, B.-M.; Deng, A.-X.; Nan, T.-G.; Li, Z.-H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79–85. [Google Scholar] [CrossRef]
- Wu, C.N.; Wang, M.Y.; Cheng, Z.H.; Meng, H.W. Response of garlic (Allium sativum L.) bolting and bulbing to temperature and photoperiod treatments. Biol. Open. 2016, 5, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, Z.; Liu, J.; Liu, F.; Zhai, R.; Zhu, C.; Wang, H.; Ma, F.; Xu, L. Histological, hormonal and transcriptomic reveal the changes upon gibberellin-induced parthenocarpy in pear fruit. Hortic. Res. 2018, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Sun, D.-W.; Blasco, J. Rapid monitoring 1-MCP-induced modulation of sugars accumulation in ripening ‘Hayward’ kiwifruit by Vis/NIR hyperspectral imaging. Postharvest Biol. Technol. 2017, 125, 168–180. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wu, Z.; Jiang, F.L. Selection and validation of garlic reference genes for quantitative real-time PCR normalization. Plant Cell Tiss. Org. 2015, 122, 435–444. [Google Scholar] [CrossRef]







| Sample Name | Raw Reads | Clean Reads | Clean Bases | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) | Total Mapped |
|---|---|---|---|---|---|---|---|---|
| Control-1 | 84,276,914 | 83,119,412 | 12.47G | 0.01 | 97.69 | 94.11 | 43.53 | 63,037,528 (75.84%) |
| Control-2 | 76,333,542 | 73,699,374 | 11.05G | 0.02 | 96.86 | 92.24 | 43.05 | 56,814,448 (77.09%) |
| Control-3 | 83,928,404 | 82,410,306 | 12.36G | 0.01 | 98.1 | 95.1 | 42.8 | 64,086,848 (77.77%) |
| GA3-1 | 83,633,550 | 82,546,464 | 12.38G | 0.01 | 97.78 | 94.28 | 43.34 | 64,036,932 (77.58%) |
| GA3-2 | 83,869,612 | 82,222,794 | 12.33G | 0.01 | 98.14 | 95.19 | 42.92 | 63,589,684 (77.34%) |
| GA3-3 | 91,727,804 | 90,117,508 | 13.52G | 0.01 | 98.16 | 95.22 | 43.05 | 69,904,142 (77.57%) |
| Unigene ID | FPKM | Log2FC | Swissport Annotation | |
|---|---|---|---|---|
| Control | GA3 Treatment | |||
| Cluster-32430.71391 | 0.86 ± 0.09 | 0.02 ± 0.05 | −4.84 | Gibberellin 20 oxidase 2 (GA20ox2) |
| Cluster-32430.95397 | 2.58 ± 0.84 | 2.84 ± 0.91 | −0.14 | Transcription factor PIF4 (PIF4) |
| Cluster-15446.0 | 0 ± 0 | 0.3 ± 0.27 | 4.91 | Cytokinin hydroxylase (CYP735) |
| Cluster-32430.98625 | 0.06 ± 0.12 | 0.44 ± 0.23 | 2.73 | Histidine kinase (AHK) |
| Cluster-32430.172148 | 13.41 ± 10.43 | 6.24 ± 2.01 | −1.10 | Auxin-induced protein (AUX) |
| Cluster-32430.118133 | 6.41 ± 1.07 | 5.11 ± 1.08 | −0.33 | Auxin response factor 19 (ARF19) |
| Cluster-32430.143582 | 16.9 ± 5.27 | 26.5 ± 13.78 | 0.78 | 6(G)-fructosyltransferase (INV) |
| Cluster-32430.190012 | 1.4 ± 0.15 | 0.23 ± 0.36 | −2.51 | Beta-glucosidase 31 (BGLU31) |
| Cluster-32430.4608 | 1.47 ± 0.76 | 0 ± 0 | −12.71 | Beta-glucosidase 25 (BGLU25) |
| Cluster-32430.132754 | 0.17 ± 0.15 | 0.74 ± 0.09 | 2.21 | 1,4-alpha-glucan-branching enzyme 3 (GBE3) |
| Cluster-49254.0 | 0.43 ± 0.12 | 0.32 ± 0.11 | −0.42 | WUSCHEL-related homeobox 3 (WOX3) |
| Cluster-32430.173042 | 5.86 ± 2.84 | 4.07 ± 1.12 | −0.53 | WUSCHEL-related homeobox 10 (WOX10) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wen, Y.; Cui, M.; Qi, X.; Deng, R.; Gao, J.; Cheng, Z. Histological, Physiological and Transcriptomic Analysis Reveal Gibberellin-Induced Axillary Meristem Formation in Garlic (Allium sativum). Plants 2020, 9, 970. https://doi.org/10.3390/plants9080970
Liu H, Wen Y, Cui M, Qi X, Deng R, Gao J, Cheng Z. Histological, Physiological and Transcriptomic Analysis Reveal Gibberellin-Induced Axillary Meristem Formation in Garlic (Allium sativum). Plants. 2020; 9(8):970. https://doi.org/10.3390/plants9080970
Chicago/Turabian StyleLiu, Hongjiu, Yanbin Wen, Mingming Cui, Xiaofang Qi, Rui Deng, Jingcao Gao, and Zhihui Cheng. 2020. "Histological, Physiological and Transcriptomic Analysis Reveal Gibberellin-Induced Axillary Meristem Formation in Garlic (Allium sativum)" Plants 9, no. 8: 970. https://doi.org/10.3390/plants9080970