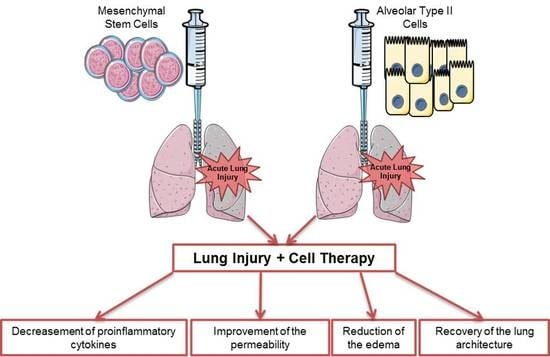
Alveolar Type II Cells or Mesenchymal Stem Cells: Comparison of Two Different Cell Therapies for the Treatment of Acute Lung Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Groups
2.3. HCl and LPS Induced ALI and Cell Infusion
2.4. Isolation and Purification of Alveolar Type II Cells
2.5. Isolation and Purification of Mesenchymal Stem Cells and Differentiation to Osteocytes, Chondrocytes, and Adipocytes
2.6. Endpoint
2.7. Bronchoalveolar Lavage Fluid Analysis
2.8. Histological Studies
2.9. Protein Extraction and Quantification from Lung Homogenates
2.10. Statistical Analysis of Results
3. Results
3.1. Effect of Both Bell Therapies in Body and Lung Weight and Bronchoalveolar Lavage Analysis
3.2. MSCs and ATII Cells Diminished Inflammation after Lung Damage
3.3. MSC and ATII Cell Therapies Improved Lung Damage and Restore Lung Architecture
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horie, S.; Gonzalez, H.E.; Laffey, J.G.; Masterson, C. Cell therapy in acute respiratory distress syndrome. J. Thorac. Dis. 2018, 10, 5607–5620. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Rocco, P.R. The potential of mesenchymal stem cell therapy for chronic lung disease. Expert Rev. Respir. Med. 2019, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Srour, N.; Thébaud, B. Mesenchymal Stromal Cells in Animal Bleomycin Pulmonary Fibrosis Models: A Systematic Review. STEM CELLS Transl. Med. 2015, 4, 1500–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukumitsu, M.; Suzuki, K. Mesenchymal stem/stromal cell therapy for pulmonary arterial hypertension: Comprehensive review of preclinical studies. J. Cardiol. 2019, 74, 304–312. [Google Scholar] [CrossRef]
- Golchin, A.; Seyedjafari, E.; Ardeshirylajimi, A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev. Rep. 2020, 16, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Laffey, J.G.; Matthay, M.A. FiftyYearsofResearchinARDS.Cell-based Therapy for Acute Respiratory Distress Syndrome. Biology and Potential Therapeutic Value. Am. J. Respir. Crit. Care Med. 2017, 196, 266–273. [Google Scholar] [CrossRef]
- Han, J.; Lu, X.; Zou, L.; Xu, X.; Qiu, H. E-Prostanoid 2 Receptor Overexpression Promotes Mesenchymal Stem Cell Attenuated Lung Injury. Hum. Gene Ther. 2016, 27, 621–630. [Google Scholar] [CrossRef]
- Cai, S.-X.; Liu, A.R.; Chen, S.; He, H.-L.; Chen, Q.-H.; Xu, J.-Y.; Pan, C.; Yang, Y.; Guo, F.-M.; Huang, Y.; et al. The Orphan Receptor Tyrosine Kinase ROR2 Facilitates MSCs to Repair Lung Injury in ARDS Animal Model. Cell Transplant. 2016, 25, 1561–1574. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gonzalez, I.; Roca, O.; Masclans, J.R.; Moreno, R.; Salcedo, M.-T.; Baekelandt, V.; Cruz, M.-J.; Rello, J.; Aran, J.M. Human Mesenchymal Stem Cells Overexpressing the IL-33 Antagonist Soluble IL-1 Receptor–Like–1 Attenuate Endotoxin-Induced Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2013, 49, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. J. Immunol. 2007, 179, 1855–1863. [Google Scholar] [CrossRef] [Green Version]
- Mei, S.H.J.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.H.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal Stem Cells Reduce Inflammation while Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, W.; Huang, S.; Tang, X.; Yao, G.; Sun, L. Mesenchymal Stem Cells Enhance Pulmonary Antimicrobial Immunity and Prevent Following Bacterial Infection. Stem Cells Int. 2020, 2020, 3169469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Krasnodembskaya, A.D.; Kapetanaki, M.; Mouded, M.; Tan, X.; Serikov, V.; A Matthay, M. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 2012, 67, 533–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavanagh, B.P.; Laffey, J.G. Cell therapy demonstrates promise for acute respiratory distress syndrome—But which cell is best? Stem Cell Res. Ther. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S. Mesenchymal Stem Cells: Angels or Demons? J. Biomed. Biotechnol. 2011, 2011, 1–8. [Google Scholar] [CrossRef] [Green Version]
- A Ankrum, J.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Mollar, A.; Nacher, M.; Gay-Jordi, G.; Closa, D.; Xaubet, A.; Bulbena, O. Intratracheal Transplantation of Alveolar Type II Cells Reverses Bleomycin-induced Lung Fibrosis. Am. J. Respir. Crit. Care Med. 2007, 176, 1261–1268. [Google Scholar] [CrossRef]
- Guillamat-Prats, R.; Gay-Jordi, G.; Xaubet, A.; Peinado, V.I.; Serrano-Mollar, A. Alveolar Type II cell transplantation restores pulmonary surfactant protein levels in lung fibrosis. J. Hear. Lung Transplant. 2014, 33, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Guillamat-Prats, R.; Puig, F.; Camprubí-Rimblas, M.; Herrero, R.; Serrano-Mollar, A.; Gómez, M.N.; Tijero, J.; Matthay, M.A.; Blanch, L.; Artigas, A. Intratracheal instillation of alveolar type II cells enhances recovery from acute lung injury in rats. J. Hear. Lung Transplant. 2018, 37, 782–791. [Google Scholar] [CrossRef]
- Chignalia, A.Z.; Vogel, S.M.; Reynolds, A.B.; Mehta, L.; Dull, R.O.; Minshall, R.D.; Malik, A.B.; Liu, Y. p120-catenin expressed in alveolar type II cells is essential for the regulation of lung innate immune response. Am. J. Pathol. 2015, 185, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Shafa, M.; Ionescu, L.I.; Vadivel, A.; Collins, J.; Xu, L.; Zhong, S.; Kang, M.; De Caen, G.; Daneshmand, M.; Shi, J.; et al. Human induced pluripotent stem cell–derived lung progenitor and alveolar epithelial cells attenuate hyperoxia-induced lung injury. Cytotherapy 2018, 20, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; E Morales, J.; Calame, D.G.; Alcorn, J.L.; A Wetsel, R. Transplantation of Human Embryonic Stem Cell–Derived Alveolar Epithelial Type II Cells Abrogates Acute Lung Injury in Mice. Mol. Ther. 2010, 18, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Sun, B. Neonatal Type II Alveolar Epithelial Cell Transplant Facilitates Lung Reparation in Piglets With Acute Lung Injury and Extracorporeal Life Support. Pediatr. Crit. Care Med. 2016, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cui, Y.; Zhou, Z.; Ding, Y.; Nie, H. Alveolar Type 2 Epithelial Cells as Potential Therapeutics for Acute Lung Injury/Acute Respiratory Distress Syndrome. Curr. Pharm. Des. 2020, 25, 4877–4882. [Google Scholar] [CrossRef]
- Guillot, L.; Nathan, N.; Tabary, O.; Thouvenin, G.; Le Rouzic, P.; Corvol, H.; Amselem, S.; Clément, A. Alveolar epithelial cells: Master regulators of lung homeostasis. Int. J. Biochem. Cell Biol. 2013, 45, 2568–2573. [Google Scholar] [CrossRef]
- Li, L.; Tian, H.; Yue, W.; Zhu, F.; Li, S.; Li, W. Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vivo. J. Cell. Physiol. 2011, 226, 1860–1867. [Google Scholar] [CrossRef]
- Miura, M.; Miura, Y.; Padilla-Nash, H.M.; Molinolo, A.A.; Fu, B.; Patel, V.; Seo, B.M.; Sonoyama, W.; Zheng, J.J.; Baker, C.C.; et al. Accumulated Chromosomal Instability in Murine Bone Marrow Mesenchymal Stem Cells Leads to Malignant Transformation. STEM CELLS 2006, 24, 1095–1103. [Google Scholar] [CrossRef]
- Hwang, N.S.; Zhang, C.; Hwang, Y.-S.; Varghese, S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009, 1, 97–106. [Google Scholar] [CrossRef]
- O’Donoghue, K.; Chan, J.; De La Fuente, J.; Kennea, N.; Sandison, A.; Anderson, J.R.; Roberts, I.; Fisk, N.M. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet 2004, 364, 179–182. [Google Scholar] [CrossRef]
- DiGirolamo, C.M.; Stokes, D.; Colter, D.; Phinney, D.G.; Class, R.; Prockop, D.J. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br. J. Haematol. 1999, 107, 275–281. [Google Scholar] [CrossRef]
- Huang, S.X.; Islam, M.N.; O’Neill, J.; Hu, Z.; Yang, Y.-G.; Chen, Y.-W.; Mumau, M.; Green, M.; Vunjak-Novakovic, G.; Bhattacharya, J.; et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol. 2013, 32, 84–91. [Google Scholar] [CrossRef] [Green Version]
- McCauley, K.; Hawkins, F.; Serra, M.; Thomas, D.C.; Jacob, A.; Kotton, D.N. Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell 2017, 20, 844–857.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamo, L.; Hibaoui, Y.; Kallol, S.; Alves, M.P.; Albrecht, C.; Hostettler, K.E.; Feki, A.; Rougier, J.-S.; Abriel, H.; Knudsen, L.; et al. Generation of an alveolar epithelial type II cell line from induced pluripotent stem cells. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L921–L932. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Ye, X.; Sun, R.; Matsumoto, Y.; Moriyama, M.; Asano, Y.; Ajioka, Y.; Saijo, Y. Differentiation of mouse induced pluripotent stem cells into alveolar epithelial cells in vitro for use in vivo. STEM CELLS Transl. Med. 2014, 3, 675–685. [Google Scholar] [CrossRef]
- Alvarez-Palomo, B.; Sanchez-Lopez, L.I.; Moodley, Y.; Edel, M.J.; Serrano-Mollar, A. Induced pluripotent stem cell-derived lung alveolar epithelial type II cells reduce damage in bleomycin-induced lung fibrosis. Stem Cell Res. Ther. 2020, 11, 213. [Google Scholar] [CrossRef]
- Serrano-Mollar, A.; Gay-Jordi, G.; Guillamat-Prats, R.; Closa, D.; Hernández-González, F.; Marin, P.; Burgos, F.; Martorell, J.; Sanchez, M.; Arguis, P.; et al. Safety and Tolerability of Alveolar Type II Cell Transplantation in Idiopathic Pulmonary Fibrosis. Chest 2016, 150, 533–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Matthay, M.; Calfee, C.S.; Zhuo, H.; Thompson, B.T.; Wilson, J.G.; Levitt, J.E.; Rogers, A.J.; Gotts, J.E.; Wiener-Kronish, J.P.; Bajwa, E.K.; et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): A randomised phase 2a safety trial. Lancet Respir. Med. 2018, 7, 154–162. [Google Scholar] [CrossRef]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.-W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, H.G.; Cruz, F.F.; Antunes, M.A.; Neto, A.V.D.M.; Oliveira, G.A.; Svartman, F.M.; Borgonovo, T.; Rebelatto, C.L.K.; Weiss, D.J.; Brofman, P.R.S.; et al. Combined Bone Marrow-Derived Mesenchymal Stromal Cell Therapy and One-Way Endobronchial Valve Placement in Patients with Pulmonary Emphysema: A Phase I Clinical Trial. STEM CELLS Transl. Med. 2016, 6, 962–969. [Google Scholar] [CrossRef]
- Stolk, J.; Broekman, W.; Mauad, T.; Zwaginga, J.; Roelofs, H.; Fibbe, W.; Oostendorp, J.; Bajema, I.; Versteegh, M.; Taube, C.; et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. Qjm: Int. J. Med. 2016, 109, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Chambers, D.C.; Enever, D.; Ilic, N.; Sparks, L.; Whitelaw, K.; Ayres, J.; Yerkovich, S.T.; Khalil, D.; Atkinson, K.M.; Hopkins, P.M. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirol. 2014, 19, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Guillamat-Prats, R.; Camprubí-Rimblas, M.; Bringué, J.; Tantinyà, N.; Artigas, A. Cell therapy for the treatment of sepsis and acute respiratory distress syndrome. Ann. Transl. Med. 2017, 5, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curley, G.F.; Hayes, M.; Ansari, B.; Shaw, G.; Ryan, A.; Barry, F.; O’Brien, T.; O’Toole, D.; Laffey, J.G. Mesenchymal stem cells enhance recovery and repair following ventilator-induced lung injury in the rat. Thorax 2011, 67, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaney, J.; Horie, S.; Masterson, C.; Elliman, S.; Barry, F.P.; O’Brien, T.; Kavanagh, B.P.; O’Toole, D.; Laffey, J.G. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 2015, 70, 625–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, L.B.; Matthay, M.A. The Acute Respiratory Distress Syndrome. New Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Spieth, P.M.; Chiumello, D.; Goyal, H.; Torres, A.; Laffey, J.G.; Hong, Y. Declining Mortality in Patients With Acute Respiratory Distress Syndrome. Crit. Care Med. 2019, 47, 315–323. [Google Scholar] [CrossRef]
- Pham, T.; Neto, A.S.; Pelosi, P.; Laffey, J.G.; De Haro, C.; Lorente, J.A.; Bellani, G.; Fan, E.; Brochard, L.J.; Pesenti, A.; et al. Outcomes of Patients Presenting with Mild Acute Respiratory Distress Syndrome. Anesthesiol. 2019, 130, 263–283. [Google Scholar] [CrossRef] [Green Version]
- Bernard, G.R.; Artigas, A. The definition of ARDS revisited: 20 years later. Intensiv. Care Med. 2016, 42, 640–642. [Google Scholar] [CrossRef] [Green Version]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Ware, L.B.; Camerer, E.; Welty-Wolf, K.; Schultz, M.J.; Matthay, M.A. Bench to bedside: Targeting coagulation and fibrinolysis in acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2006, 291, L307–L311. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; Network, N.A. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Slutsky, A.S. Precision medicine for cell therapy in acute respiratory distress syndrome. Lancet Respir. Med. 2019, 7, e13. [Google Scholar] [CrossRef] [Green Version]
- Puig, F.; Herrero, R.; Guillamat-Prats, R.; Gómez, M.N.; Tijero, J.; Chimenti, L.; Stelmakh, O.; Blanch, L.; Serrano-Mollar, A.; Matthay, M.A.; et al. A new experimental model of acid- and endotoxin-induced acute lung injury in rats. Am. J. Physiol. Cell. Mol. Physiol. 2016, 311, L229–L237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huppert, L.A.; Matthay, M.A.; Ware, L.B. Pathogenesis of Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2019, 40, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rodriguez, E.; Gay-Jordi, G.; Mucci, A.; Lachmann, N.; Serrano-Mollar, A. Lung surfactant metabolism: Early in life, early in disease and target in cell therapy. Cell and Tissue Research 2016, 367, 721–735. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Sun, Y.; Yi, X.; Wei, X.; Liu, W.; Zhang, Q.; Yi, H.; Chen, G. Mesenchymal stromal cells ameliorate acute lung injury induced by LPS mainly through stanniocalcin-2 mediating macrophage polarization. Ann. Transl. Med. 2020, 8, 334. [Google Scholar] [CrossRef]
- Chaleshtori, S.S.; Dezfouli, M.R.M.; Fakhr, M.J. Mesenchymal stem/stromal cells: The therapeutic effects in animal models of acute pulmonary diseases. Respir. Res. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Rabani, R.; Volchuk, A.; Jerkic, M.; Ormesher, L.; Garces-Ramirez, L.; Canton, J.; Masterson, C.; Gagnon, S.; Tatham, K.C.; Marshall, J.; et al. Mesenchymal stem cells enhance NOX2-dependent reactive oxygen species production and bacterial killing in macrophages during sepsis. Eur. Respir. J. 2018, 51, 1702021. [Google Scholar] [CrossRef]
- Shologu, N.; Scully, M.; Laffey, J.G.; O’Toole, D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int. J. Mol. Sci. 2018, 19, 2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. STEM CELLS 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbs, L.G. Isolation and culture of alveolar type II cells. Am. J. Physiol. Cell. Mol. Physiol. 1990, 258, L134–L147. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Score Per Field |
|---|---|
| Haemorrhage | 0–1 |
| Peribronchial infiltration | 0–1 |
| Interstitial edema | 0–2 |
| Pneumocyte hyperplasia | 0–3 |
| Intraalveoalr infiltration | 0–3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillamat-Prats, R.; Camprubí-Rimblas, M.; Puig, F.; Herrero, R.; Tantinyà, N.; Serrano-Mollar, A.; Artigas, A. Alveolar Type II Cells or Mesenchymal Stem Cells: Comparison of Two Different Cell Therapies for the Treatment of Acute Lung Injury in Rats. Cells 2020, 9, 1816. https://doi.org/10.3390/cells9081816
Guillamat-Prats R, Camprubí-Rimblas M, Puig F, Herrero R, Tantinyà N, Serrano-Mollar A, Artigas A. Alveolar Type II Cells or Mesenchymal Stem Cells: Comparison of Two Different Cell Therapies for the Treatment of Acute Lung Injury in Rats. Cells. 2020; 9(8):1816. https://doi.org/10.3390/cells9081816
Chicago/Turabian StyleGuillamat-Prats, Raquel, Marta Camprubí-Rimblas, Ferranda Puig, Raquel Herrero, Neus Tantinyà, Anna Serrano-Mollar, and Antonio Artigas. 2020. "Alveolar Type II Cells or Mesenchymal Stem Cells: Comparison of Two Different Cell Therapies for the Treatment of Acute Lung Injury in Rats" Cells 9, no. 8: 1816. https://doi.org/10.3390/cells9081816