Abstract
Phosphorus (P) loss is undesirable but prevalent in agricultural fields, as its leaching into natural water bodies usually triggers algae blooms and hypoxic conditions hazardous to aquatic ecosystems. In many developing countries, animal manure, which usually contains high levels of N, P, and organic matter, is directly applied as an economic fertilizer. While the manure-related greenhouse gas (GHG) emission issues have been widely discussed, the impacts of manure applications on nutrient leaching has been rarely discussed. In this study, we have investigated the impact of humic acid (HA) (i.e., a major derivative during manure degradation) on the adsorption and transport of P in goethite-coated silica sand through a series of batch isotherms and column experiments under steady-state flow conditions. The results show that 40 mg L−1 HA could lower the maximum adsorption capacity of goethite-coated silica (for P) by ~ 60%, leading to an increase in P leaching by ~ 70% in the media. This research demonstrates the negative impact that HA applications may have on P retention in agricultural fields.
Similar content being viewed by others
1 Introduction
Concerns over excessive eutrophication have been increasingly reported (Fischer et al. 2017). As a consequence of high nutrient (especially phosphorus (P)) accumulation, eutrophication provokes nuisance algae blooms and “dead zoom” in aquatic ecosystem. Particular attentions have been made on appreciable subsurface P loss from agricultural land, which was well documented in coarse-textured soil, high weathered soil, and porous organic soil (Kleinman et al. 2015). Thus, it is an exigency to understand P transport in subsurface soil and subsequent leaching to surrounding water bodies.
Phosphorus transport is largely affected by soil structure and properties. Although metal oxides (e.g., magnetite, kyanite, and goethite) in soil enhance nutrients’ stability (Yaghi and Hartikainen 2013), macropores and preferential flow in subsurface soil provide direct pathways for P leaching (Bergström et al. 2015). An example is colloid-facilitated transport, a strong sorbent bonding P, would rapidly transfer P through the macropores to aquatic environment (Vendelboe et al. 2011).
To improve P acquisition, inorganic P fertilizers were reported to be co-applied with novel materials such as artificial humic substances (Yang et al. 2019), natural humic substances (Hua et al. 2008), and geochemical-modified sewage sludge (Zhang et al. 2020). Besides these materials, animal manure is always considered as an economical alternative to enhance P bioavailability (Shafqat and Pierzynski 2013). Manure is well known for its high proportion of nutrients and organic matter. But direct application of manure left severe environmental footprints, such as greenhouse gas emission and groundwater pollution (Chadwick et al. 2011). Thus, before application, composting is highly recommended to stabilize and sanitize the manure (Ren et al. 2018). The amount of humic acid (HA) in manure compost is a major indicator of product quality and biological maturity (Moral et al. 2009). And a high-quality manure compost exhibits an enrichment of soil HA and reduction of water-extracted organic acid in the soil biota (Bernal et al. 2009). It acts as a slow-release fertilizer because the humidification prevents nutrient loss from mineralization (Mulbry et al. 2005).
However, a significant role of HA on P loss has been largely underestimated, especially when mineral oxides extensively covered the soil. HA competes with nutrients for bonding receptors on mineral oxides through its functional groups (e.g., carboxylic acids, carbonyl, and phenolic hydroxyl groups) (Niu et al. 2011). In Fu et al.’s study, higher affinity was found between soil HA and synthetic goethite than P and goethite (Fu et al. 2013). Thus, soil retention capacity of nutrients would be deteriorated by soil HA and mineral oxide interaction. However, there is still a handful of studies that investigated P dynamic transport and retention under such HA and mineral-rich media.
In order to better understand the underlying mechanisms of low utilization efficiency and high leachability of P, we exanimated phosphate (PO43−) transport and adsorption in the soil environment by column and bench experiments. It is hypothesized that (1) HA (from soil natural material and decomposed manure) in the soil environment would simultaneously bind to inorganic soil surface; (2) the HA coating would eliminate the receptor sites on soil surface that were originally available for PO43−; (3) the application of HA could accelerate the P leaching in subsurface soil.
2 Materials and Methods
2.1 Sample Preparation
The silica sand (8 mesh) in this study was obtained from Fisher Scientific Inc. (Hampton, NH), which had a diameter of 2.36 mm, porosity of 0.4, and a surface area of 3.02 ± 0.02 m2 g−1 measured by BET gas adsorption (ASAP2010, Micromeritics, Norcross, GA). Silica sand was treated successively with sodium acetate, hydrogen peroxide, sodium dithionate, and sodium citrate to remove organic matter. Then it was saturated with Na+ using 1 M phosphate-buffered saline (pH 7.0). Treated silica sand had a bulk density of 1.48 g cm−3 and a cation exchange capacity of 15.2 cmol kg−1.
Goethite was prepared referring to previous study (Schwertmann et al. 1985). Briefly, after aging of 21 days at 25 °C, the suspension of the mixture of 1.0 M ferric nitrate and 1.0 M potassium hydroxide (1:9, v/v) was washed carefully with de-ionized water via centrifugation. The rinsed solid was then re-suspended in 0.4 M hydrochloride acid. After washed and dialyzed against de-ionized water, it was freeze-dried to obtain crystalline goethite. To coat silica sand, goethite was mixed with silica sand (1:10, 1:5, and 1:3, w/w) in 0.01 M sodium nitrate solution (pH 7.5) and shaken for 48 h. Coated silica sand was washed with 0.1 M sodium nitrate (pH 7.0) via centrifugation. After rinsed with de-ionized (DI) water, coated silica sand was oven-dried at 110 °C. In the study, X-ray photoelectron spectroscopy (XPS) analysis was used for determining the efficiency of goethite coating on silica sand. The X-ray tube setting was 60 kV and 55 mA. The quantification of goethite coating was determined by dissolving coated silica sand in nitric acid (95%) and hydrofluoric acid (40%) (2:1, v/v).
With the verbal permission from the farm owner, dairy manure was collected from a dairy farm located one mile south away from Mayo, Florida, for HA extraction (for research purposes only). The manure was mixed with DI water. And the pH of the mixed solution was adjusted to 12 with sodium hydroxide. After shaking for 1 h, the manure solution was filtered by a 0.45-μm filter. The pH of the filtrate was lowered with hydrochloride acid. Then the precipitates were collected and dried as extracted HA. HA was dissolved by de-ionized water to the concentrations of 0, 10, 20, and 40 mg L−1 for the following experiments. HA solutions have been used for both adsorption isotherm experiments and transport column experiments. Concentrations were quantified by UV-vis spectrometry (Agilent Technologies Cary 60) at a wavelength of 254 nm. The spectroscopic measurements were calibrated with a TOC analyzer (Shimadzu TOC 5000).
2.2 Adsorption Isotherm Experiments
Batch adsorption isotherms were conducted to examine P in the form of PO43− adsorption on goethite-coated silica sand following the procedures described in a previous study (Li et al. 2018). Estimating from previous studies, the commonly used range of HA is 0–20 mg L−1 (a typical soil bulk density = 1.6 g cm−3 was used for unit conversion) (Adani et al. 1998; Ferrara and Brunetti 2008; Tahir et al. 2011). In order to thoroughly estimate the effect of HA, we have also included the bad organic management using excessive HA (i.e., HA > 20 mg L−1). A series of 25 mL vials containing solutions (20 mL) with P (0–200 mg L−1) in the form of monopotassium phosphate (KH2PO4, pH = 7) and 4 g goethite-coated silica sand (HA was in range of 0–40 mg L−1) as well as blank controls (sealed with Teflon-lined screw caps) were agitated on a Wrist Action Shaker (Burrell Scientific, Model 75) to reach equilibrium. The suspension was then centrifuged at 12,000×g for 15 min, after which, PO43− concentration in the supernatant was measured by ionic chromatography. The amount of P adsorbed on goethite-coated silica sand was calculated based on the mass balance of the difference between the initial and equilibrated solutions. Three replicates were conducted for each sample.
In this research, P had Langmuir adsorption isotherms on goethite-coated silica sand. When equilibrium was reached, PO43− adsorption on goethite-coated silica sand was described as (Adachi et al. 1997):
where S is the adsorbed P on goethite-coated silica sand; qmax is the maximum mass of P required to form a mono-layer coverage of the media surface; Ce is equilibrium P concentration; and K is the Langmuir adsorption constant.
2.3 Phosphorus Transport Column Experiments
Column experiments were conducted to study the impact of HA and mineral coating on the transport of anionic P in porous media. The column experiments were conducted under saturated conditions with the column (5 cm ID × 15 cm length) vertically oriented. The bottom of column was sealed with a custom fit to permit the flow of water and retain the silica sand. To pack the column with goethite-coated silica sand, the column was first filled with nanopure DI water to a height of 2 to 3 cm and the media were packed in the column through CO2 solvation to eliminate air pockets. A height of 2 to 3 cm of nanopure de-ionized water was maintained.
Before starting each experiment, approximately 10 pore volumes of nanopure de-ionized water (ionic strength adjusted with NaNO3 to 0.001 M) was eluted through the column to stabilize the column condition by a peristaltic pump (Masterflex, Cole-Parmer, Vernon Hills, IL). A conservative pulse tracer (chloride) breakthrough curve was generated separately before the introduction of P solution. For each experiment, 2 pore volumes of sample solution which contained K2HPO4 solution (50 mg L−1, pH = 7) and HA (0, 10, 20, and 40 mg L−1) were introduced into the column at a flow rate of 2.5 mL min−1. The column was then continuously flushed with nanopure DI water (ionic strength adjusted with NaNO3 to 0.001 M) until the background signal was detected by a fraction collector. HA and P in elution were measured by ionic chromatography and UV-vis spectrometry, separately. For each column experiment, it was duplicated for three times. And the inconsistency of breakthrough curves was within 5% (95% CI).
Phosphorus transport in goethite-coated silica sand was described by the equilibrium-kinetic two-site model (Toride et al. 1995):
where C1 and C2 are the dimensionless P concentration in the solution and on the media surface, respectively; β is the partition coefficient; R is the retardation factor (\( R=1+\frac{\uprho_{\mathrm{b}}{K}_{\mathrm{d}}}{\uptheta} \), where ρb is the media bulk density, Kd is the partition coefficient of P between the solution and media, and θ is the porosity); T is the dimensionless time; P is the Peclet number (\( \mathrm{P}=\frac{vL}{D} \), where v is the interstitial pore-water velocity, L is the length of column, and D is the dispersion coefficient); Z is the dimensionless axial coordinate; ω is the dimensionless mass transfer coefficient; and μ1 and μ2 are the first-order dimensionless deposition coefficient in the solution and on the media surface, respectively. For this research, it was assumed that the dominating P deposition occurred in the solution. Thus, μ2 was set to 0.
At the same time, a pulse-type boundary condition was used for the upper boundary and a zero gradient was assumed for the lower boundary (Yuan et al. 2017):
where q is the specific discharge (Darcian fluid flux); t0 is the duration of injection; and C0 is the initial PO43− concentration. Transport parameters in Eqs. (2) and (3) were obtained by fitting the experimentally obtained breakthrough data using an implicit, finite difference scheme of CXTFIT (Toride et al. 1995). All the parameters were optimized by minimizing the sum of squared differences between observed and fitted concentrations using the nonlinear least square method (Toride et al. 1995).
2.4 Statistical Analysis
The experimental data were processed and analyzed by one-way one-factor analysis of variance (ANOVA) using SPSS for Windows 14.0 (IBM, Armonk, NY). The corresponding Scheffé test was made to differentiate means with 95% confidence (p < 0.05) when the study of variance showed differences between means.
3 Results
3.1 Goethite-Coated Silica Sand Characterization
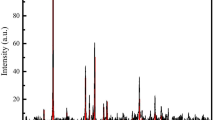
Different goethite/silica sand ratio (i.e., w/w ratio of 1:10, 1:5, and 1:3) led to variable goethite coating on silica sand. The corresponding goethite-coated silica sand samples are named as GS1, GS2, and GS3. The XPS results indicated an Fe peak at the energy level of 6.4 keV (Fig. 1). The peak was most pronounced for GS3 and least pronounced for GS1, with GS2 in between. Goethite coating of GS1, GS2, and GS3 was determined to be 7.5, 10.2, and 14.1 mg Fe g−1 sand, respectively. There was no obvious change in particle diameter and bulk density after goethite coating. However, after goethite coating, the surface area (S0) increased from 3.02 ± 0.02 to 4.91 ± 0.04, 6.78 ± 0.10, and 7.17 ± 0.84 m2 g−1, and the cation exchange capacity (CEC) decreased from 15.2 to 12.4, 9.75, and 7.21 cmol kg−1 for GS1, GS2, and GS3, respectively.
3.2 Phosphorous Adsorption Isotherm on Goethite-Coated Silica Sand
Phosphorous adsorption isotherms on the goethite-coated silica sand in the absence and presence of HAs are demonstrated in Fig. 2. The adsorption of anionic P on GS1, GS2, and GS3 increased with the increase of the equilibrium concentration. P had the greatest adsorption on GS3, followed by GS2 and GS1, while HA limited the PO43− adsorption to different degrees. In this study, P exhibited Langmuir adsorption isotherms on goethite-coated silica sand. Great agreements between experimental data and simulated curves were indicated by the prediction coefficient R2 and significant coefficient p value noted in Fig. 2. Higher HA concentration generally led to a lower adsorption capacity of the media. This investigation agreed with previous assumption about the influence of HA.
3.3 Phosphorous Transport and Leachability in Goethite-Coated Silica Sand
Tracer (Cl−) transport was studied before P transport experiments in goethite-coated silica sand (i.e., G1, G2, and G3). Nearly all the input tracer was eluted from the column. Since tracer should not be retarded or retained in the media, the retardation factor was set to 1.0 (i.e., Kd = 0), and the deposition coefficients (i.e., μ1 = 0, μ2 = 0) were set to zero during the simulation. Dispersion coefficient D was thus determined and used for the following interpretation of P transport in the corresponding media.
Breakthrough curves of P transport in goethite-coated silica sand were characterized by a self-sharpening front, which became broader and diffuser at the elution limb (Fig. 3). The long-lasting tails indicated kinetic-controlled PO43− retention in the columns. Retardation was exhibited as the leading edge dominated by the reversible adsorption of PO43−. Decreased retardation was found when the concentration of HA was higher. In addition, negative charged P displayed irreversible adsorption or kinetic adsorption in goethite-coated silica sand, which was evidenced by the reduced mass recovery during the transport. P breakthrough curves were fitted well with the model. The accuracy of transport modeling was expressed by the sum of the squared differences which ranges from 0.92 to 0.99.
4 Discussion
4.1 Influence of Goethite Coating on Phosphorus Retention
After coating with goethite, S0 and CEC changed linearly with the increased goethite dosage in the samples. CEC is an essential soil property in terms of nutrient management. Soil CEC is affected by soil properties such as parent materials, organic matters, and pH. Surface soil and wetland soil always exhibited large CEC because of intense reducing conditions and high dissolved organic matters (Guedes et al. 2015). In this study, reduced CEC in higher mineral-coated sand is caused by the neutralization of the charges through coating reaction (Scheidegger et al. 1993). Increased PO43− adsorbency and decreased CEC corresponded to the increasing dosage of Fe oxide, when HA = 0 mg L−1. At the same time, larger S0 was observed on GS2 and GS3. It has been well-estimated from previous study that the binding capacity of Fe oxides is proportional to its specific surface area (Gérard 2016). Thus, higher dosages of goethite in GS2 and GS3 contribute to the larger surface areas, which further provide abundant binding receptors for P adsorption to take place (Wang et al. 2012).
Parameter qmax, μ1, and R derived from Eqs. (1) and (2) indicate the goethite altered the soil properties (Fig. 4). Parameter qmax of GS3 appeared slightly ahead of GS2, but much higher than GS1 when HA = 0 mg L−1. The changes of qmax were correlated to the adsorption capacity changes of soil media. The phenomenon demonstrates that mineral coating efficiently trapped the mobile nutrients. μ1 demonstrates that P deposition on GS1 is less than GS2 and GS3. Parameter R derived from column experiments reveals a heavily retarded transport on GS3, which is also corresponding to its largest S0. Retardation increases the possibility of plant uptake during the P transport. When comparing simulated parameters (i.e., qmax, μ1, and R) of three samples, it is noticeable that higher goethite content significantly increased the retardation as well as the adsorption capacity. This conclusion is consistent with previous researches. Studies confirmed that the ability of soil constituents to bind PO43− is dominantly contributed to Fe/Al oxide and clay mineral (Gérard 2016; Zavaschi et al. 2020). In Bortoluzzi et al.’s study, Fe/Al sesquioxide distribution controls soil P sorption capacities regardless of the environmental gradient conditions (Bortoluzzi et al. 2015). Maluf et al. verified that a higher proportion of Fe2O3 and Al2O3 in Oxisol leads to a higher P adsorbency than the Entisol (Maluf et al. 2018).
a Retardation factor R as the function of humic acid, b deposition coefficient μ1 as the function of humic acid, and c maximum mass required to form a mono-layer coverage qmax as the function of humic acid, applying to GS1 (goethite/silica sand w/w = 1/10), GS2 (goethite/silica sand w/w = 1/5), and GS3 (goethite/silica sand w/w = 1/3), respectively (p < 0.01)
4.2 Influence of HA on Phosphorus Transport and Loss in Soil
In the breakthrough curve (Fig. 3), retardation (resulted from the equilibrium adsorption) is exhibited by the delay of the curve, i.e., the ratio of solution velocity to that of PO43−. Retention and adsorption (resulted from kinetic adsorption) are presented by the reduced amplitudes, i.e., the ratio of the eluted PO43− to that of the input. HA causes a significant impairment on soil absorbency and retention, especially for GS3 (Fig. 3). The capacity of anionic P retention on GS3 was reduced ~ 70%. But the retardation was not severely infected. A clearer picture is revealed from Fig. 4 b and c; qmax and μ1 were all largely slumped after adding HA, while the changes of R were less pronounced after adding HA, as a flatter slope indicated in Fig. 4 a.
The possible interactions between HA, mineral oxides, and P have also been discussed in previous studies by mathematical models, filed investigations, and batch experiments (Fu et al. 2013; Gerke 2010; Wang et al. 2015). In this study, P affinity is weakened after HA-mineral binding formed, as the qmax was decreased by ~ 75% adding 40 mg L−1 of HA. Similarly, Wang et al. observed that less amount of PO43− was adsorbed on HA-coated ferrihydrite than the pure ferrihydrite (Wang et al. 2015). At the same time, less isoelectric point and less specific surface area were found on HA-coated ferrihydrite than pure ferrihydrite. The formation of bidentate ligands between PO43− and Fe oxide is the dominant mechanism estimated by attenuated total reflectance Fourier-transform infrared spectroscopy in their study.
The impacts of HA on P were various in previous studies, because of different soil properties, forms of nutrient, and even the extraction methods (Mumbach et al. 2020; Zavaschi et al. 2020). For example, Fu et al. verified higher P adsorption onto goethite was achieved with a lower pH either with the presence or absence of HA (Fu et al. 2013). Different absorbencies resulted from the formation of different ligands (i.e., monodentate and bidentate ligands) during pH variations. Borie et al. found that volcanic soil retained high humic substance as well as stable mineral-humus complexes (i.e., allophane, imogolite, Al- and Fe-humus complexes) (Borie et al. 2019). High organic content in volcanic soil reforms the accumulated P. More than 65% of soil P, which was previously claimed only bound to mineral, was linked to organic substances. da Rocha et al. concluded that P extracted from pasture was more recalcitrant to plant than that from agroforestry and forestry (Rocha Junior et al. 2018). Some studies determined that HA could be beneficial to the P bioavailability if managed properly. For example, HA significantly promoted the labile fractions of P from single superphosphate incubation in clayey soil and sandy soil (de Jesus Souza et al. 2019). HA-coated monoammonium phosphate (MAP) fertilizer retained more mobile P than pure MAP in mineral-rich soil (do Nascimento et al. 2018). This was because of the chelating function of HA. It combines cationic nutrients like Ca2+ and Fe3+, which simultaneously allows the inner P fertilizer to dissolve without precipitation or adsorption. Fink et al. derived the conclusion that organic matters only affect the P transport by retardation but not retention capacity of soil in the literature review (Fink et al. 2016). However, soil retention capacity and retardation were both changed after adding HA in this study (Fig. 3 and Fig. 4a). Similar conclusion was drawn, which indicated the mineral oxide coating has larger positive effect than the deterioration caused by HA on P adsorption (Yan et al. 2016). These various conclusions indicate that the largely unknown dynamic nutrient transport in the subsurface soil still existed.
5 Conclusions
The dynamic interaction among phosphorus (P), humic acid (HA), and mineral oxides has been clearly revealed in this study. Mineral oxides altered soil properties and then increased phosphate (PO43−) adsorption capacity, evidencing from the enlarged surface area and limited cation exchange capacity. However, the binding sites on mineral oxides preferred the ligand formation with HA to P. Minimal PO43− retention was observed in column experiments when HA was 40 mg L−1, indicating the majority of adsorption sites were covered by HA. The bioavailability of adsorbed P via monodentate and/or bidentate ligands is doubted, which reveals further investigation is needed. It can be concluded from this study that a HA-rich soil is not a guarantee for P-efficient utilization, especially when composted manure is applied. The economic benefits may not overcome the environmental footprint caused by such agricultural community. Therefore, it is an urgency for regulating HA-involved fertilizers.
References
Adachi S, Panintrarux C, Matsuno R (1997) Methods for estimating the parameters of nonlinear adsorption isotherms of Langmuir and Freundlich types from a response curve of pulse input of an adsorbate. Biosci Biotechnol Biochem 61:1626–1633. https://doi.org/10.1271/bbb.61.1626
Adani F, Genevini P, Zaccheo P, Zocchi G (1998) The effect of commercial humic acid on tomato plant growth and mineral nutrition. J Plant Nutr 21:561–575. https://doi.org/10.1080/01904169809365424
Bergström L, Kirchmann H, Djodjic F, Kyllmar K, Ulén B, Liu J, Andersson H, Aronsson H, Börjesson G, Kynkäänniemi P, Svanbäck A, Villa A (2015) Turnover and losses of phosphorus in Swedish agricultural soils: long-term changes, leaching trends, and mitigation measures. J Environ Qual 44:512–523. https://doi.org/10.2134/jeq2014.04.0165
Bernal MP, Alburquerque JA, Moral R (2009) Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour Technol 100:5444–5453. https://doi.org/10.1016/j.biortech.2008.11.027
Borie F, Aguilera P, Castillo C, Valentine A, Seguel A, Barea JM, Cornejo P (2019) Revisiting the nature of phosphorus pools in Chilean volcanic soils as a basis for Arbuscular Mycorrhizal Management in plant P acquisition. J Soil Sci Plant Nutr 19:390–401. https://doi.org/10.1007/s42729-019-00041-y
Bortoluzzi EC, Pérez CAS, Ardisson JD, Tiecher T, Caner L (2015) Occurrence of iron and aluminum sesquioxides and their implications for the P sorption in subtropical soils. Appl Clay Sci 104:196–204. https://doi.org/10.1016/j.clay.2014.11.032
Chadwick D, Sommer S, Thorman R, Fangueiro D, Cardenas L, Amon B, Misselbrook T (2011) Manure management: implications for greenhouse gas emissions. Anim Feed Sci Technol 166-167:514–531. https://doi.org/10.1016/j.anifeedsci.2011.04.036
de Jesus Souza B, Lopes do Carmo D, Santos RHS, de Oliveira TS, Fernandes RBA (2019) Residual contribution of green manure to humic fractions and soil fertility. J Soil Sci Plant Nutr 19:878–886. https://doi.org/10.1007/s42729-019-00086-z
do Nascimento CAC, Pagliari PH, Faria LDA, Vitti GC (2018) Phosphorus mobility and behavior in soils treated with calcium, ammonium, and magnesium phosphates. Soil Sci Soc Am J 82:622–631. https://doi.org/10.2136/sssaj2017.06.0211
Ferrara G, Brunetti G (2008) Influence of foliar applications of humic acids on yield and fruit quality of table grape cv. Italia. OENO One 42:79–87. https://doi.org/10.20870/oeno-one.2008.42.2.822
Fink JR, Inda AV, Tiecher T, Barrón V (2016) Iron oxides and organic matter on soil phosphorus availability. Ciênc agrotec 40:369–379. https://doi.org/10.1590/1413-70542016404023016
Fischer P, Pöthig R, Venohr M (2017) The degree of phosphorus saturation of agricultural soils in Germany: current and future risk of diffuse P loss and implications for soil P management in Europe. Sci Total Environ 599:1130–1139. https://doi.org/10.1016/j.scitotenv.2017.03.143
Fu Z, Wu F, Song K, Lin Y, Bai Y, Zhu Y, Giesy JP (2013) Competitive interaction between soil-derived humic acid and phosphate on goethite. Appl Geochem 36:125–131. https://doi.org/10.1016/j.apgeochem.2013.05.015
Gérard F (2016) Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils — a myth revisited. Geoderma 262:213–226. https://doi.org/10.1016/j.geoderma.2015.08.036
Gerke J (2010) Humic (organic matter)-Al(Fe)-phosphate complexes: an underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci 175:417–425. https://doi.org/10.1097/SS.0b013e3181f1b4dd
Guedes RS, Fernandes AR, Santos de Souza E, Rosa e Silva JR (2015) Maximum phosphorus adsorption capacity adjusted to isotherm models in representative soils of eastern Amazon. Commun Soil Sci Plant Anal 46:2615–2627. https://doi.org/10.1080/00103624.2015.1089264
Hua Q-X, Li J-Y, Zhou J-M, Wang H-Y, Du C-W, Chen X-Q (2008) Enhancement of phosphorus solubility by humic substances in ferrosols1 1project supported by the National Natural Science Foundation of China (no. 30400273) and the Potash and Phosphate Institute/Potash and Phosphate Institute of Canada (PPI/PPIC). Pedosphere 18:533–538. https://doi.org/10.1016/S1002-0160(08)60044-2
Kleinman PJ, Church C, Saporito LS, McGrath JM, Reiter MS, Allen AL, Tingle S, Binford GD, Han K, Joern BC (2015) Phosphorus leaching from agricultural soils of the Delmarva Peninsula, USA. J Environ Qual 44:524–534. https://doi.org/10.2134/jeq2014.07.0301
Li SM, Barreto V, Li RW, Chen G, Hsieh YP (2018) Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J Anal Appl Pyrolysis 133:136–146. https://doi.org/10.1016/j.jaap.2018.04.010
Maluf HJGM, Silva CA, Curi N, Norton LD, Rosa SD (2018) Adsorption and availability of phosphorus in response to humic acid rates in soils limed with CaCO3 or MgCO3. Ciênc agrotec 42:7–20. https://doi.org/10.1590/1413-70542018421014518
Moral R, Paredes C, Bustamante M, Marhuenda-Egea F, Bernal M (2009) Utilisation of manure composts by high-value crops: safety and environmental challenges. Bioresour Technol 100:5454–5460. https://doi.org/10.1016/j.biortech.2008.12.007
Mulbry W, Westhead EK, Pizarro C, Sikora L (2005) Recycling of manure nutrients: use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresour Technol 96:451–458. https://doi.org/10.1016/j.biortech.2004.05.026
Mumbach GL, Gatiboni LC, Dall’Orsoletta DJ, Schmitt DE, Pessotto PP, de Oliveira CMB (2020) Phosphorus extraction with soil test methods affected by soil P sorption capacity. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-020-00259-1
Niu H, Zhang D, Zhang S, Zhang X, Meng Z, Cai Y (2011) Humic acid coated Fe3O4 magnetic nanoparticles as highly efficient Fenton-like catalyst for complete mineralization of sulfathiazole. J Hazard Mater 190:559–565. https://doi.org/10.1016/j.jhazmat.2011.03.086
Ren X, Awasthi MK, Wang Q, Zhao J, Li R, Tu Z, Chen H, Awasthi SK, Zhang Z (2018) New insight of tertiary-amine modified bentonite amendment on the nitrogen transformation and volatile fatty acids during the chicken manure composting. Bioresour Technol 266:524–531. https://doi.org/10.1016/j.biortech.2018.07.010
Rocha Junior PRD, Ribeiro PH, Mesquita LF, Andrade FV, Mendonça EDS (2018) Distribution of C and inorganic phosphorus fractions in different aggregate sizes under forestry, agroforestry system and pasture. J Soil Sci Plant Nutr 18:361–375. https://doi.org/10.4067/S0718-95162018005001202
Scheidegger A, Borkovec M, Sticher H (1993) Coating of silica sand with goethite: preparation and analytical identification. Geoderma 58:43–65. https://doi.org/10.1016/0016-7061(93)90084-X
Schwertmann U, Cambier P, Murad E (1985) Properties of goethites of varying crystallinity. Clay Clay Miner 33:369–378. https://doi.org/10.1346/CCMN.1985.0330501
Shafqat MN, Pierzynski GM (2013) Soil test phosphorus dynamics in animal waste amended soils: using P mass balance approach. Chemosphere 90:691–698. https://doi.org/10.1016/j.chemosphere.2012.09.050
Tahir MM, Khurshid M, Khan MZ, Abbasi MK, Kazmi MH (2011) Lignite-derived humic acid effect on growth of wheat plants in different soils. Pedosphere 21:124–131. https://doi.org/10.1016/S1002-0160(10)60087-2
Toride N, Leij F, Van Genuchten MT (1995) The CXTFIT code for estimating transport parameters from laboratory or field tracer experiments
Vendelboe AL, Moldrup P, Heckrath G, Jin Y, de Jonge LW (2011) Colloid and phosphorus leaching from undisturbed soil cores sampled along a natural clay gradient. Soil Sci 176:399–406. https://doi.org/10.1097/SS.0b013e31822391bc
Wang X, Zhang L, Zhang H, Wu X, Mei D (2012) Phosphorus adsorption characteristics at the sediment–water interface and relationship with sediment properties in FUSHI reservoir, China. Environ Earth Sci 67:15–22. https://doi.org/10.1007/s12665-011-1476-z
Wang H, Zhu J, Fu Q, Hu H (2015) Adsorption of phosphate on pure and humic acid-coated ferrihydrite. J Soils Sediments 15:1500–1509. https://doi.org/10.1007/s11368-015-1095-5
Yaghi N, Hartikainen H (2013) Enhancement of phosphorus sorption onto light expanded clay aggregates by means of aluminum and iron oxide coatings. Chemosphere 93:1879–1886. https://doi.org/10.1016/j.chemosphere.2013.06.059
Yan J, Jiang T, Yao Y, Lu S, Wang Q, Wei S (2016) Preliminary investigation of phosphorus adsorption onto two types of iron oxide-organic matter complexes. J Environ Sci (China) 42:152–162. https://doi.org/10.1016/j.jes.2015.08.008
Yang F, Zhang S, Song J, Du Q, Li G, Tarakina NV, Antonietti M (2019) Synthetic humic acids solubilize otherwise insoluble phosphates to improve soil fertility. Angew Chem Int Ed 58:18813–18816. https://doi.org/10.1002/anie.201911060
Yuan H, Li S, Liu J, Song C, Chen G (2017) Cry1Ab adsorption and transport in humic acid-coated geological formation of alumino-silica clays. Water Air Soil Pollut 228. https://doi.org/10.1007/s11270-017-3579-y
Zavaschi E, de Abreu FL, Ferraz-Almeida R, do Nascimento CAC, Pavinato PS, Otto R, Vitti AC, Vitti GC (2020) Dynamic of P flux in tropical acid soils fertilized with humic acid–complexed phosphate. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-020-00265-3
Zhang S, Du Q, Cheng K, Antonietti M, Yang F (2020) Efficient phosphorus recycling and heavy metal removal from wastewater sludge by a novel hydrothermal humification-technique. Chem Eng J 394:124832. https://doi.org/10.1016/j.cej.2020.124832
Funding
The work was supported by National Natural Science Foundation of China (Grant No. 41572295) and Youth Innovation Promotion Association CAS (No. 2015272 and 2017376). This work was also supported by USDA-NIFA Grant No. 2016-67020-25275 and Department of Energy Minority Serving Institution Partnership Program (MSIPP) managed by the Savannah River National Laboratory under SRNS contract DE-AC09-08SR22470.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Y., Li, S. & Chen, G. Impact of Humic Acids on Phosphorus Retention and Transport. J Soil Sci Plant Nutr 20, 2431–2439 (2020). https://doi.org/10.1007/s42729-020-00308-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-020-00308-9