Abstract
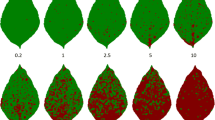
Anthracnose is one of the most destructive grapevine diseases in warm and humid regions, but no efficient tools are available to quantify disease symptoms in different parts of the plant. Therefore, this study aimed to develop and validate standard area diagrams (SADs) to evaluate grapevine anthracnose on the fruit and shoot. For fruit SAD development, fruit clusters showing signs of anthracnose symptoms were sampled from five different white grape genotypes: Aromera, Bronner, GF24, Felicia and Helios. To develop the shoot SAD, plants from a population that segregated for anthracnose resistance were artificially inoculated. Fruits and shoots with characteristic symptoms were photographed, and a total of 30 and 31 images, respectively, were selected to develop each SAD. The SADs from fruit and shoots included severity ranging from 0.5 to 17.6% and from 0.8 to 45.9%, respectively. In order to verify the applicability of both SADs, random raters evaluated the images with and without the use of the SAD. Rater data were utilized for validation of both SADs using linear regression, absolute error and Lin’s statistic, and repeatability was tested by inter-rate analysis. The developed SADs improved the accuracy and repeatability among the raters. In addition, diagrammatic scales for anthracnose severity on the fruit and shoot decreased absolute error and disease overestimation. In conclusion, the use of both sets of SADs improved grapevine anthracnose evaluation on the fruit and shoots.




Similar content being viewed by others
References
Angelotti F, Scapin CR, Tessmann DJ, Vida JB, Oliveira RR, Canteri MG (2008) Diagrammatic scale for assessment of grapevine rust. Trop Plant Pathol 33(6):439–443
Azevedo de Paula PV, Pozza EA, Santos LA, Chaves E, Maciel MP, Paula JCA (2016) Diagrammatic scales for assessing brown eye spot (Cercospora coffeicola) in red and yellow coffee cherries. J Phytopathol 164(10):791–800. https://doi.org/10.1111/jph.12499
Barros LB, Biasi LA, Carisse O, De Mio LLM (2015) Incidence of grape anthracnose on different VITIS labrusca and hybrid cultivars and rootstocks combination under humid subtropical climate. Australas Plant Pathol 44(4):397–403. https://doi.org/10.1007/s13313-015-0353-8
Bock CH, Hotchkiss MW, Wood BW (2016) Assessing disease severity: accuracy and reliability of rater estimates in relation to number of diagrams in a standard area diagram set. Plant Pathol 65(2):261–272. https://doi.org/10.1111/ppa.12403
Bock CH, Poole GH, Parker PE, Gottwald TR (2010) Plant disease severity estimated visually by digital photography and image analysis and by hyperspectral imaging. Crit Rev Plant Sci 29(2):59–107. https://doi.org/10.1080/07352681003617285
Braga ZV, Santos RF, Amorim L, Appezzato-da-Glória B (2019) Histopathology of infection and colonisation of Elsinoë ampelina on grapevine leaves. Eur J Plant Pathol 154(4):1009–1019. https://doi.org/10.1007/s10658-019-01721-2
Buffara CRS, Angelotti F, Vieira RA, Bogo A, Tessmann DJ, Bem BPD (2014) Elaboration and validation of a diagrammatic scale to assess downy mildew severity in grapevine. Cienc Rur 44(8):1384–1391. https://doi.org/10.1590/0103-8478cr20131548
Clive JW (1971) An illustrated series of assessment keys for plant diseases, their preparation and usage. Edit. WL Seaman. Dis Survey, Canadian, pp 1–39
Costa AP, Pires MDC, Peixoto JR, Blum LEB, Faleiro FG (2018) Standard area diagram set for bacterial spot assessment in fruits of yellow passion fruit. Rev Bras Frut 40(6):1–15. https://doi.org/10.1590/0100-29452018039
Del Ponte EM, Pethybridge SJ, Bock CH, Michereff SJ, Machado FJ, Spolti P (2017) Standard area diagrams for aiding severity estimation: scientometrics, pathosystems, and methodological trends in the last 25 years. Phytopathol 107(10):1161–1174. https://doi.org/10.1094/PHYTO-02-17-0069-FI
Delrot S, Grimplet J, Carbonell-Bejerano P, Schwandner A, Bert PF, Bavaresco L, Costa LD, Di Gaspero G, Duchêne E, Hausmann L, Malnoy M, Morgante M, Ollat N, Pecile M, Vezzulli S (2020) Genetic and genomic approaches for adaptation of grapevine to climate change. In: Kole C (ed) Genomic designing of climate-smart fruit crops. Springer, Cham, pp 157–270
Dolinski MA, Duarte HDSS, da Silva JB, De Mio LLM (2017) Development and validation of a standard area diagram set for assessment of peach rust. Eur J Plant Pathol 148(4):817–824. https://doi.org/10.1007/s10658-016-1138-9
Domiciano GP, Duarte HSS, Moreira EN, Rodrigues FA (2014) Development and validation of a set of standard area diagrams to aid in estimation of spot blotch severity on wheat leaves. Plant Pathol 63(4):922–928. https://doi.org/10.1111/ppa.12150
Eichhorn KW, Lorenz DH (1984) Phaenologische Entwicklungsstadien der Rebe. Eur Med Plant Prot Org 14:295–298
Ellis MA, Erincik O (2008) Anthracnose of grape. Agriculture and Natural Resources. The Ohio State University. https://ohiograpeweb.cfaes.ohio-state.edu/sites/grapeweb/files/imce/pdf_factsheets/Anthracnose.pdf. Accessed 10 February 2020
Fan XL, Barreto RW, Groenewald JZ, Bezerra JDP, Pereira OL, Cheewangkoon R, Mostert L, Tian CM, Crous PW (2017) Phylogeny and taxonomy of the scab and spot anthracnose fungus Elsinoë (Myriangiales. Dothideomycetes). Stud Mycol 87:1–41. https://doi.org/10.1016/j.simyco.2017.02.001
Fantin LH, Braga K, Canteri MG, Dias AR, Borges EP (2018) Development and validation of diagrammatic scale to assess target spot severity in cotton. Australas Plant Pathol 47(5):491–497. https://doi.org/10.1007/s13313-018-0576-6
Gamer M, Lemon J, Fellows I, Singh P (2012) irr: Various coefficients of interrater reliability and agreement. R package version 0.84. Accessed 10 March 2019
González-Domínguez E, Martins RB, Del Ponte EM, Michereff SJ, García-Jiménez J, Armengol J (2014) Development and validation of a standard area diagram set to aid assessment of severity of loquat scab on fruit. Eur J Plant Pathol 139(2):419–428. https://doi.org/10.1007/s10658-014-0400-2
Guginski-Piva CA, Bogo A, Gomes BR, Menon JK, Nodari RO, Welter LJ (2018) Morphological and molecular characterization of Colletotrichum nymphaeae and C. fructicola associated with anthracnose symptoms of grape in Santa Catarina state. Southern Brazil. J Plant Dis Prot 125(4):405–413. https://doi.org/10.1007/s41348-018-0176-2
Hopkins DL, Harris JW (2000) A greenhouse method for screening grapevine seedlings for resistance to anthracnose. HortScience. https://doi.org/10.21273/HORTSCI.35.1.89
IPGRI OIV (1997) Descriptors for grapevine (Vitis spp.). International Union for the Protection of New Varieties of Plants, Geneva, Switzerland/Office International de la Vigne et du Vin, Paris, France/International Plant Genetic Resources Institute, Rome, Italy 142(34):1–4
Kassemeyer HH (2017) Fungi of grapes. In: König H, Unden G, Fröhlich J (eds) Biology of microorganisms on grapes, in must and in wine. Springer, Cham, pp 103–132
Li Z, Fan Y, Chang P, Gao L, Wang X (2020) Genome sequence resource for Elsinoë ampelina, the causal organism of grapevine anthracnose. Mol Plant-Micr Inter 33(4):576–579. https://doi.org/10.1094/MPMI-12-19-0337-A
Li Z, Zhang S, Han R, Zhang H, Li K, Wang X (2019) Infection process and host responses to Elsinoë ampelina, the causal organism of grapevine anthracnose. Eur J Plant Pathol 155(2):571–582. https://doi.org/10.1007/s10658-019-01793-0
Lin LIK (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45:255–268
Liu M, Zhang W, Zhou Y, Liu Y, Yan JY, Li XH, Hyde KD (2016) First report of twig anthracnose on grapevine caused by Colletotrichum nymphaeae in China. Plant Dis 100(12):2530–2530. https://doi.org/10.1094/PDIS-05-16-0632-PDN
Murria S, Kaur N, Arora A, Arora NK (2018) Biochemical characterization of superior seedless variety of grape (Vitis vinifera L.) for resistance to anthracnose. Ind Phytopathol 71(3):399–405. https://doi.org/10.1007/s42360-018-0051-x
Nita M, Ellis MA, Madden LV (2003) Reliability and accuracy of visual estimation of Phomopsis leaf blight of strawberry. Phytopathol 93(8):995–1005. https://doi.org/10.1094/PHYTO.2003.93.8.995
OIV - International Organisation of Vine and Wine (2017) World VitiViniculture situation. OIV Statistical Report on World Vitiviniculture. www.oiv.int/public/medias/5479/oiv-en-bilan-2017
Pedroso C, Lage DAC, Henz GP, Café-Filho AC (2011) Development and validation of a diagrammatic scale for estimation of anthracnose on sweet pepper fruits for epidemiological studies. J Plant Pathol 93(1):219–225 https://www.jstor.org/stable/41998960
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci Discuss 4 (2):439. https://hal.archives-ouvertes.fr/hal-00298818
Pirrello C, Mizzotti C, Tomazetti TC, Colombo M, Bettinelli P, Prodorutti D, Peressotti E, Zulini L, Stefanini M, Angeli G, Masiero S, Welter L, Hausmann L, Veyulli S (2019) Emergent Ascomycetes in viticulture: an interdisciplinary overview. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01394,10
Ponti L, Gutierrez A, Boggia A, Neteler M (2018) Analysis of grape production in the face of climate change. Climate 6(2):20. https://doi.org/10.3390/cli6020020
Poolsawat O, Tharapreuksapong A, Wongkaew S, Chaowiset W, Tantasawat P (2012) Laboratory and field evaluations of resistance to Sphaceloma ampelinum causing anthracnose in grapevine. Australas Plant Pathol 41(3):263–269. https://doi.org/10.1007/s13313-012-0127-5
Core Team R (2017) R: A Language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria http://www.r-project.org
Sachet MR, Citadin I, Danner MA, Guerrezi MT, Pertille RH (2017a) DiseasePlan-a spreadsheet application for training people to assess disease severity and to assist with standard area diagram development. Cienc Rur 47(6):e20160924. https://doi.org/10.1590/0103-8478cr20160924
Sachet MR, Danner MA, Citadin I, Pertille RH, Guerrezi MT (2017b) Standard area diagram set for olive leaf spot assessment. Cienc Rur 47(6):e20160923. https://doi.org/10.1590/0103-8478cr20160923
Santos PHDD, Mussi-Dias V, Freire MDGM, Carvalho BM, Silveira SFD (2017) Diagrammatic scale of severity for postharvest black rot (Ceratocystis paradoxa) in coconut palm fruits. Sum Phytopathol 43(4):269–275. https://doi.org/10.1590/0100-5405/170792
Santos RF, Ciampi-Guillardi M, Amorim L, Massola Júnior NS, Spósito MB (2018) Aetiology of anthracnose on grapevine shoots in Brazil. Plant Pathol 67(3):692–706. https://doi.org/10.1111/jph.12675
Santos RF, Spósito MB (2018) Improving assessments of anthracnose severity on grapevine leaves through the development of a standard area diagram set. Australasian Plant Pathol 47(4):357–364. https://doi.org/10.1007/s13313-018-0566-8
Silva HFD, Pinto KMS, Nascimento LCD, Silva ECD, Souza WCOD (2019) Evaluation of the use of biotic and abiotic resistance elicitors against anthracnose in grapevine (Vitis labrusca L.). Sum Phytopathol 45(1):70–75. https://doi.org/10.1590/0100-5405/180414
Sompong M, Wongkaew S, Tantasawat P, Buensanteai N (2012) Morphological. Pathogenicity and virulence characterization of Sphaceloma ampelinum the causal agent of grape anthracnose in Thailand. Afr J Micro Res 6(10):2313–2320
Stevenson M, Nunes T, Heuer C, Marshall J, Sanchez J, Thornton R et al. (2017) epiR: tools for the analysis of epidemiological data. R package version 0.9–87. Accessed 10 March 2019
Töpfer R, Hausmann L, Harst M, Maul E, Zyprian E, Eibach R (2011) New horizons for grapevine breeding. In: Hanke MV (ed) Flachowsky H. Global Science Books, Methods in Temperate Fruit Breeding, pp 79–100
Vale FXR, Filho EIF, Liberato JR, Zambolim L (2001) QUANT – a software to quantify plant disease severity. In: Proceedings of the international workshop on plant disease epidemiology 2001. Ouro Preto, Brazil, pp 1–160
Wang YJ, Liu YL, He PC, Lamikanra O, Lu J (1998) Resistance of Chinese Vitis species to Elsinoë ampelina (de Bary) shear. HortScience 33(1):123–126
Welter LJ, Göktürk-Baydar N, Akkurt M, Maul E, Eibach R, Töpfer R, Zyprian EM (2007) Genetic mapping and localization of quantitative trait loci affecting fungal disease resistance and leaf morphology in grapevine (Vitis vinifera L). Mol Breed 20(4):359–374. https://doi.org/10.1007/s11032-007-9097-7
Welter LJ, Grando MS, Zyprian E (2016) Basics of grapevine genetic analysis. In Genetics, Genomics, and Breeding of Grapes, CRC Press, pp 165–187
Yadav NVS, de Vos SM, Bock CH, Wood BW (2013) Development andvalidation of standard area diagrams to aid assessment of pecan scabsymptoms on fruit. Plant Pathol 62(2):325–335. https://doi.org/10.1111/j.1365-3059.2012.02641.x
Yan JY, Jayawardena MMRS, Goonasekara ID, Wang Y, Zhang W, Liu M, Bahkali A (2015) Diverse species of Colletotrichum associated with grapevine anthracnose in China. Fung Diver 71(1):233–246. https://doi.org/10.1007/s13225-014-0310-9
Acknowledgments
We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for scholarship to LRM (Finance Code 001), to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support (Proj 423476/2018-1) and scholarship to RON and DMS, and to the Fundação de Amparo à Pesquisa e Inovação de Santa Catarina (FAPESC) for financial support (Proj. TO2017 TR1844). We thankful for photographer Augusto Marques taking high quality photos of anthracnose symptoms. We also are grateful to Dr. Ricardo Feliciano dos Santos for kindly providing us the E. ampelina isolate AVBR118.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modesto, L.R., Steiner, D.R.M., Menon, J.K. et al. Standard area diagram set for anthracnose severity on grapevine bunches and shoots. Australasian Plant Pathol. 49, 561–569 (2020). https://doi.org/10.1007/s13313-020-00728-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-020-00728-2