Abstract
In this study, the scales of Micropogonias undulatus fish were investigated as precursors for the development of low-cost biosorbent for the removal of Pb(II) and Zn(II) from aqueous media. The biosorbent was characterised using Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM) and X-ray diffractometry (XRD). Adsorption parameters (temperature, biosorbent dosage and contact time) were optimised using response surface methodology Box–Behnken experimental design. The optimal factors for Zn(II) removal by croaker fish scale were 145.5 min, 7.01 g/L biosorbent dosage, 30 °C and pH 5.4. The optimal factors for the removal of Pb(II) were 179.3 min, 6.61 g/L biosorbent dosage, 20 °C and pH 3.4. The numerical optimisation revealed that the optimal removal efficiency for Zn(II) and Pb(II) sorption is 96.45% and 98.76%, respectively. The biosorption of both heavy metals was best fit to Freundlich isotherm and pseudo-second-order kinetic models. Thermodynamics studies revealed that the biosorption process was exothermic and spontaneous.
Similar content being viewed by others
Introduction
Pollution of water bodies by industrial effluents has been a long-standing environmental problem globally (Babatunde et al. 2019). This effluent has been established to contain heavy metals, dyes, pharmaceutically active compounds and a plethora of other inorganic pollutants that need to be mitigated in a variety of ways (Chandrakanth et al. 2014; Eletta et al. 2019). Heavy metals are non-biodegradable and could be toxic to living organisms (Abbas et al. 2014) and even in little concentrations can be very toxic to human (Alluri et al. 2007). One of the techniques in wastewater treatment by the adsorption of pollutants is the utilisation of living and non-living biomass in a process known as biosorption (Demirbas 2008). Biosorption can be defined as the removal of substances from a solution by biological material (Adeniyi and Ighalo 2019; Goeffrey 2008). This technology has been extensively investigated in the removal of heavy metals (Ighalo and Adeniyi 2020a). Techniques and mechanisms involved in the process have been examined (Amirnia 2015; Ramachandra et al. 2006). Bioaccumulation (Zabochnicka-Świątek and Krzywonos 2014) describes an active process in which pollutant uptake involves the metabolic activity of a living organism (Abdi and Kazemi 2015).
Fish scales have also been studied as a source of biosorbents for heavy metal removal (Eletta and Ighalo 2019). Scales from fish species such as Gadus morhua (Basu et al. 2006, 2007; Rahaman et al. 2015), Tilapia nilotica (Huang 2007; Zhe et al. 2014; Zhu et al. 2013), Oreochromis niloticus (Neves et al. 2017; Ribeiro et al. 2015; 2018a, b), Labeo rohita (Chakraborty et al. 2012; Iqbal et al. 2011; Kondapalli and Mohanty 2011; Mandal et al. 2015; Marrakchi et al. 2016, 2017; Nadeem et al. 2008) and Catla catla (Das et al. 2016; Kondapalli and Mohanty 2011; Prabu et al. 2012; Srividya and Mohanty 2009) have been studied for the adsorption of pollutants from aqueous media. Croaker fish (Micropogonias undulatus) is scarcely investigated, and it is one easy way to assess in the current demographic of this study. Croaker fish (Micropogonias undulatus) is readily available in Nigeria, and the scale is a waste material with no other competitive use. It has also not been reportedly studied as a biosorbent for heavy metals (Ighalo and Eletta 2020). This leaves an interesting knowledge gap to explore. Studies such as this are justified in the light of the need for waste valorisation and environmental protection.
This paper was targeted at a specific environmental problem. The levels of heavy metal pollution from industrial effluent are a significant issue especially in the demographic of the study. The choice of heavy metal was made based on the observations from real water samples. The aim of this study is to investigate the viability of the Micropogonias undulatus (croaker) scales as a precursor in developing efficient and cost-effective biosorbents for the removal of Zn(II) and Pb(II) from aqueous solutions and optimise the parameters by Box–Behnken design. The biosorbent will be characterised using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and X-ray diffractometry (XRD). Response surface methodology and Box–Behnken design will be used to optimise the biosorption process. Equilibrium modelling alongside kinetic and thermodynamic studies will also be conducted.
Materials and methods
Preparation of biosorbent
Micropogonias undulatus (croaker) scales were sourced from the market in Ilorin metropolis in Kwara State, Nigeria. The scales were washed thoroughly with water and liquid detergent to remove oil and other surface contamination. This was followed by liberal rinsing with distilled water. The scales were pre-treated by soaking in 5% Nitric acid (HNO3) in a plastic container for 24 h. This was to ensure that compounds like iron and lead oxides (FeO, PbO) are removed, thereby increasing the adsorption capacity (Othman et al. 2016). The scale was further soaked in distilled water for another 24 h and then dried in an oven (GenLab Electric oven model; MINO/50) at 60 °C until they were crispy and constant in weight. The dry scales were ground to a fine powder in an electric blender and sieved through 100 mesh to achieve 150 µm maximum particle size.
Characterisation of biosorbent
The biosorbent was characterised by Fourier transform infrared spectroscopy (FTIR), scanning electron microscope (SEM) and X-ray diffractometry (XRD). FTIR (Shimadzu, FTIR-8400S, Japan) was performed according to the description by Adeniyi et al. (2020). SEM (Phenom ProX, Eindhoven Netherlands) was performed based on the description in Adeniyi et al. (2019). For the XRD analyses, an Empyrean X-ray diffractometer (Pan Analytical) was employed to investigate the physical properties of the adsorbent as it relates to the crystallinity of the material. The XRD was operated with Cu Kα emission (ƛ = 1.54105 Ǻ, 45 kV, 40 mA per s).
Determination of target pollutant
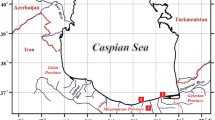
The specific environmental problem leading to the choice of target pollutant was obtained by assessing the heavy metal amount in a pharmaceutical effluent in Ilorin Nigeria. The wastewater was sourced from a pharmaceutical company located at 8o28′N, 4o33′E in Ilorin, Nigeria. The satellite image in Fig. 1 shows the vicinity around which the wastewater was sourced. The point marked with a white cross-hair represents the place of release of effluents from the nearby pharmaceutical industry. The water is released and fed into the tributary which goes on to join the Asa River on the east. The area in consideration can be observed to be urban. There are numerous negative environmental impacts of such effluents released into the river.
The heavy metal concentration in the wastewater was analysed using BUCK scientific ACCUSYS 211 Atomic Absorption Spectrophotometer (AAS). The wastewater sample was digested to make the heavy metals easily detectable during analysis. HNO3 and HCl (in the ratio 3:1) making 12 mL was dissolved in 2 mL of the wastewater sample in a beaker. The resultant solution was heated directly (in a fume cupboard) till only white fumes were evolved, and the solution was clear. The solution was filtered with Whatman filter paper, and the filtrate was made up to 50 mL using distilled water. The solution was analysed AAS, and the results are shown in Table 1. It was observed that zinc (Zn) and lead (Pb) appeared at or above the WHO standard. Hence, it can be stated that the wastewater is a source of heavy metal pollutants into the ecosystem in Ilorin town. From the findings of the wastewater characterisation, target impurities for this study were chosen as zinc and lead since they are above the WHO standard. The pH of the wastewater was measured and found to be 6.7.
Preparation of stock solutions
All reagents used in this study were analytical grade (> 99.9% purity). The reagents used were nitric acid (HNO3), hydrochloric acid (HCl), zinc sulphate (ZnSO4·7H2O), lead nitrate (Pb(NO3)2) and distilled water (H2O). For the preparation of the stock solution of Zn(II), 4.396 g of ZnSO4·7H2O was dissolved in 1 L of distilled water to give a 1000 mg/L of Zn(II). For the stock solution of Pb(II), 1.5985 g of Pb(NO3)2 was dissolved in 1 L of distilled water to give a 1000 mg/L solution.
Batch biosorption experiments
All biosorption experiments will be performed in 250-mL glass-stoppered Erlenmeyer flasks with 50 ml of wastewater solution. A weighed amount of biosorbent (in grams) was added to the water to form a solution of known concentration or dosage. The pH of the solution was left at their natural value of 3.4 for the lead solution and 5.4 for the zinc solution without any adjustments. The flasks were agitated at a constant speed of 120 rpm for a known length of time ‘t’ hours in an incubator shaker based on the experimental design in a constant temperature incubator shaker. The incubator shaker was set at a known temperature based on the experimental design. At the attainment of the required contact time, the samples were taken off and filtered through Whatman filter papers. The samples obtained are then digested and analysed by Atomic Absorption Spectrophotometry (ACCUSYS 211). The optimisation experiment in the current paper was developed using response surface methodology (RSM), and the design matrix chosen was Box–Behnken design (BBD). BBD is a three-level optimisation design on response surface methodology. The factors to be optimised were adsorbent dosage (1–5 g/L), agitation time (10–100 min) and temperature (20–40 °C). An experimental plan for the optimisation totalling 17 runs was generated by the software Design Expert 10.0.1 (shown in the supplementary data). For the equilibrium isotherm study, the temperature was set at 30 °C, initial metal concentration (Ci) was 1000 mg/L and contact time of 120 min will be used. The adsorbent dosage was varied from 2 to 10 g/L in increments of 2. For the kinetic studies, the temperature was set at 30 °C, initial metal concentration (Ci) was at 1000 mg/L and dosage of 6 g/L was utilised. The contact time was varied from 30 to 150 min in increments of 30. For experiments, the pH was maintained at their initial values of 5.4 and 3.4 Zn(II) and Pb(II) biosorption. For the thermodynamic studies, the response surface optimal for Zn(II) equilibrium concentration will be obtained at 20, 30 and 40 °C and 5.5 g/L dosage and 95 min.
Results and discussion
Biosorbent characterisation
The FTIR was conducted to know the functional groups present in the biosorbent and to ascertain those responsible for the sorption of Zn(II) and Pb(II) (summarised in Table 2). Figure 2 shows the FTIR spectrum of fish scale before adsorption, then after the adsorption of Pb(II) and Zn(II), respectively. Spectrum A shows the presence of carbonyl group C=O at peak 1643 cm−1, while the peak at 1049 cm−1 corresponds to ethers (C–O stretch) (Othman et al. 2016). The peak observed at 1535 cm−1 correspond to the presence of nitro compounds which does not shift frequency in the spectra of the fish scale loaded with Pb(II) and Zn(II) which implies that the nitro compounds may not be involved in the adsorption. Studies have shown the shifting of frequency level of many functional groups to different levels after adsorption which indicates the possible involvement of the functional groups for the uptake of metal cations (Chowdhury et al. 2012; Othman et al. 2016). Changes in the intensity and shifts in the position of peaks were observed in the spectra after Pb(II) and Zn(II) adsorption on the fish scales. There was no shifting observed in the peak at 1643 cm−1 which characterise carbonyl group stretch (C=O) from ketone, carboxylic acid and aldehyde (Othman et al. 2016; Pavia et al. 2008). The characteristic peak at 1774 cm−1 also suggests the presence of C=O in the adsorbent (Spectrum A). However, the peak remains unchanged after Pb(II) adsorption, while it shifts to about 1736 cm−1 and increases in intensity after Zn(II) adsorption.
The peak at 2924 cm−1 corresponds to the presence of amine group N–H in the adsorbent. The peak shifts to 2939 cm−1 in the Pb(II) adsorption, while it remains unchanged but increases in intensity after Zn(II) adsorption. The small peak observed at 1427 cm−1 in the raw fish scale spectra may correspond to the C–C stretch of aromatic rings, which further shifts to 1435 cm−1 and 1450 cm−1 in the spectra for fish scale loaded with Pb(II) and Zn(II), respectively. This implies that the aromatic C–C group was involved in the adsorption of both Pb(II) and Zn(II) (Zayadi and Othman 2013a). The peak 1049 cm−1 observed in spectrum A shifted to 1018 cm−1 and 1026 cm−1 in spectra B and C, respectively, and may correspond to C–O stretch of esters and carboxylic acids (Chowdhury et al. 2012; Zayadi and Othman 2013b). The adsorbent characterisation proved the involvement of carbonyl group (carboxylic acid, ketone and aldehyde), nitro compounds, aromatic C–C and amine group in the adsorption of Zn(II) and Pb(II) ions on fish scales as also noted by Othman et al. (2015).
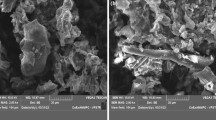
Figure 3 is the SEM image of the fish scale biosorbent. A large pebble-like structure, agglomerated with some grit —grain particles—can be observed. Also observed was a mushroom-like cluster, embedded in a similar but cubic shaped structure. However, clouded white pyramid forms were observed randomly on the morphological surface of the sample. The adsorbent has a generally heterogeneous surface which makes it very suitable for use as an adsorbent (Ighalo and Adeniyi 2020b). The XRD spectrum (shown in the supplementary data) shows that the inorganic content of the croaker fish scale is majorly Brushite (CAS:01-072-0713). The common name is calcium hydrogen phosphate hydrate (CaHPO4·H2O2). The crystallographic parameters from the analysis revealed that the particles are monoclinic and have a density of 2.32 g/cm3. The crystallographic parameters are a = 5.812 Å, b = 15.18 Å and c = 6.239 Å. The sharp peaks indicate the crystalline nature of the material.
Optimisation and modelling studies
Box–Behnken experimental design in response surface methodology was used for the optimisation studies. The goals of optimisation were to maximise the uptake of heavy metals and to do the numerical optimisation in range of the factors studied (Ighalo and Adeniyi 2020d). It was observed that the optimal factors for Zn(II) removal by croaker fish scale are 145.5 min, 7.01 g/L biosorbent dosage and temperature of about 30 °C. The optimal factors for the removal of Pb(II) by croaker fish scales are 179.3 min, 6.61 g/L biosorbent dosage and temperature of 20 °C. The optimum removal efficiency for Pb(II) was marginally higher than that of Zn(II). The removal efficiencies were excellent, and these go to prove that croaker scales are excellent adsorbents for the removal of Zn(II) and Pb(II) from polluted effluents. The results obtained are for pH of 5.4 for Zn(II) and 3.4 for Pb(II). From the information on removal efficiencies obtained, response surface models were developed by the software. These models are entirely empirical and can be used to make predictions/estimations of Zn(II) and Pb(II) removal efficiency at a given set of factor levels. Based on the input data, the software predicted a quadratic model as best fit for Zn(II) sorption and linear model as best fit for Pb(II) sorption. An analysis of variance (ANOVA) was computed and presented in the supplementary data.
From ANOVA in the supplementary data for Zn(II), we observe that the model is significant for predicting the removal efficiency of Zn(II) by croaker fish scales based on a given level of input factors. Dosage and time make significant contributions to the model, but the temperature does not. The final equation in terms of actual factors best fitting the experimental result is presented in Eq. 1.
where A is the temperature in °C, B is the biosorbent dosage in g/L and C is the contact time in min. From the ANOVA in the supplementary data for Pb(II), it can be seen that the model is significant and suitable for making estimations of lead biosorption based on the factor inputs. Time was the only significant factor in the model, however. The linear model obtained from the software in terms of actual factors is presented in Eq. 2.
where A is the temperature in °C, B is the biosorbent dosage in g/L and C is the contact time in min.
Parametric studies for Zn(II) biosorption
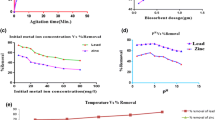
Response surface plots of a combination of the factors were also generated to elucidate any combinatorial effects of the factors on the amount of Zn(II) removed. The factors considered were adsorbent dosage, contact time and temperature. There are three possible 2-factor combinations of a set of three factors, hence three response surface plots. Figure 4a, b does not show significant effects of each factor on each other. The convolutions of Fig. 4c reveal that a greater shaking time will lead to a higher amount adsorbed, and this relationship holds in all domain of dosage. The surface is oriented in such a way that increased removal efficiency with increasing dosage is also noticeable.
An initial increase in removal efficiency was observed with an increase in temperature (Fig. 4a). However, upon the exceeding of a maximum of about 30 °C, a drop in biosorption was noticed. From careful observation of the y-axis, it can be opined that the effect of temperature on the process is only marginal. This is a key pointer towards a physical adsorption process where the effect of temperature is less pronounced and higher temperature tends to reduce the amount adsorbed. The amount of Zn(II) adsorbed increased with increasing dosage. This is expected as a higher dosage indicates the presence of a greater number of active sites available for adsorption. The trend was observed over the entire range of the factor studied. It can also be observed that the amount adsorbed increases steadily with contact time. Maximum adsorption is observed at about 145 min of equilibration. This trend is because at lesser shaking time, equilibrium will not have been achieved; hence, the transfer of adsorbate from the solution to the adsorbent surface would not have been completed, leading to a lesser removal efficiency (Eletta et al. 2020).
Parametric studies for Pb(II) biosorption
Response surface plots of a combination of the factors were also generated and are presented in Fig. 5 to elucidate any combinatorial effects of the factors on the amount of Pb(II) removed. The plots show that there are no significant factor interactions in the biosorption process.
Figure 5 reveals that the amount of Pb(II) removed by adsorption reduces with an increase in temperature. This informs that the adsorption process is likely to be strongly exothermic. This assertion was confirmed by the thermodynamics study where the enthalpy was shown to be − 10.28 kJ/mol. The amount of Pb(II) adsorbed increased with increasing dosage. This is expected as a higher dosage indicates the presence of a greater number of active sites available for adsorption. An optimum of about 7 mg/L was observed beyond which there was no longer any significant improvement in the removal efficiency. It can also be observed that the amount adsorbed increases steadily with shaking time. This was observed in the entire domain of the factor level studied. This is because at lesser shaking time, equilibrium will not have been achieved; hence, the transfer of adsorbate from the solution to the adsorbent surface would not have been completed leading to a lower metal uptake.
Equilibrium isotherm modelling
The results of experiments for equilibrium concentration of Zn(II) biosorption were fitted into Langmuir, Freundlich and Temkin isotherms. The respective plots for the different isotherms were done with Microsoft Excel to determine the trend line, and linear regression expression was also shown (in the supplementary data). From these, we can obtain values for the slope and intercepts which were needed for computing the different isotherm parameters. The coefficient of determination (R2) informs us to what extent the variability of the data is captured by the model. The R-squared values of the different plots reveal that the Freundlich isotherm (R2 = 0.9685) is the best fit for the biosorption of Zn(II) onto croaker fish scales. The intercepts and slopes were used to compute the other isotherm parameters, and the results are presented in Table 3. The value of the constant n is greater than 1. This informs that the process is a physical adsorption process. The monolayer adsorption capacity of Zn(II) biosorption was 555.6 mg/g. The results of equilibrium isotherm experiments for Pb(II) biosorption were also fitted into the Langmuir, Freundlich and Temkin isotherms. The respective plots for the different isotherms were done with Microsoft Excel (shown in the supplementary data). The trend line and linear regression expression were also obtained and were for determining the slope and intercepts which were important for computing the different isotherm parameters. The R-squared values of the different plots reveal that the Freundlich isotherm (R2 = 0.8903) is the best fit for the biosorption of Pb(II) onto croaker fish scales. The intercepts and slopes were used to compute the other isotherm parameters, and the results are presented in Table 3. The value of the constant n is greater than 1. This informs that the process is a physical adsorption process and that the adsorption is generally favourable. The monolayer adsorption capacity for Pb(II) biosorption was 909.9 mg/g.
From the postulates of the Freundlich isotherm, the following can be deduced about the adsorption process. The uptake of metal ions occurs on a heterogeneous surface by multilayer adsorption. The mechanisms of sorption of the adsorbate to the adsorbent are not singular, and the energies of the forces of attraction can differ from one adsorbed molecule to the other. This is also expected as surface sorption is accompanied by sorption into interstitials on the macro-molecular structure of the bio-material. Based on the postulates of the Freundlich isotherm, we can also assume that the stronger binding sites are occupied first and the binding strength decreases with increasing degree of site occupation. There are also possible interactions between already adsorbed molecules.
Kinetics modelling
The results of biosorption kinetics were computed and fitted into the pseudo-first-order, pseudo-second-order and intra-particle diffusion models (shown in the supplementary data). From the R2 values, it can be surmised that the model of best fit is the pseudo-second-order model (R2 = 1). This has also been observed in numerous other fish scale biosorption studies (Eletta and Ighalo 2019). The slopes and intercepts were used to compute the other kinetic parameters (presented in Table 4). It can also be surmised that the model of best fit for Pb(II) sorption is the pseudo-second-order model (R2 = 0.9957). The slopes and intercepts were used to compute the other kinetic parameters (Table 4). The rate of Zn(II) biosorption is 0.0041 g/mg min−1. The value of qe calculated from the second-order model is quite close to those obtained from experiments. From the postulates of the pseudo-second-order model, we can, therefore, state that Zn(II) biosorption by croaker scales is dependent on the number of metal ions present in the solution as well as the free biosorption sites on the biosorbent surface. The rate of Pb(II) biosorption is 0.0009 g/mg min−1. The value of qe calculated from the second-order model is quite close to those obtained from experiments. From the R2 values, we can conclude that the sorption of Pb(II) by croaker fish scale is also according to second-order kinetics. This has also been observed in numerous other fish scale biosorption studies (Eletta and Ighalo 2019).
Biosorption thermodynamics studies
The Gibbs free energy change (ΔG) values can discern whether a process is spontaneous is not, and the negative values of ΔG imply a spontaneous process. The enthalpy change (ΔH) provides information about the exothermic nature or endothermic nature of the process and differentiate between the physical and chemical adsorption process (Ighalo and Adeniyi 2020c). The intercepts and slopes of van’t Hoff’s plots (not shown) were used to compute the thermodynamic parameters. The results obtained are presented in Table 5.
We see that the value of Gibbs free energy is negative for all temperatures. This informs that the process is spontaneous. Once the biosorbent is added to a solution containing the pollutants and agitated, adsorption will take place. The negative values of the enthalpies indicate that the process is exothermic. This is supported by the decrease in metal uptake with increased temperature. However, the biosorption of Pb(II) is more strongly exothermic than that of Zn(II). The negative entropy for Pb(II) indicates less randomness in the system, hence more energy requirement for sorption (in the form of agitation). The positive entropy for Zn(II) indicates more randomness in the system, hence less energy requirement for sorption. This is expected as Pb(II) is a heavier ion than Zn(II).
Comparison with other biosorbents
Table 6 compares the adsorption capacity obtained in the current study for Zn(II) and Pb(II), respectively, to those obtained for other bio-materials. It can be seen that the croaker fish scale is an excellent biosorbent for the removal of these heavy metals from aqueous solutions. Croaker fish scale was only better than tea leaves for Zn(II) biosorption, and it was the highest of the compared bio-materials for Pb(II) biosorption.
Conclusion
The scales of croaker fished were investigated as precursors for the development of low-cost adsorbents capable of removing heavy metals from aqueous solutions. Several key findings were made by this research work. SEM revealed the morphology of the particles to be large pebble-like structure agglomerated with some grit—grain particles. XRD showed that the sample fish scale majorly consists of Brushite with a common name of calcium hydrogen phosphate hydrate (CaHPO4·H2O2). The crystallographic parameters revealed that it is monoclinic and has a density of 2.32 g/cm3. The FTIR analysis of the used biosorbent revealed that aromatic C–C group and C–O stretch of esters and carboxylic acids were involved in the absorption of both Pb(II) and Zn(II). The optimal factors for Zn(II) removal by croaker fish scale were 145.5 min, 7.01 g/L biosorbent dosage, 30 °C and pH 5.4. The optimal factors for the removal of Pb(II) were 179.3 min, 6.61 g/L biosorbent dosage, 20 °C and pH 3.4. The numerical optimisation revealed that the optimal removal efficiency for Zn(II) and Pb(II) sorption is 96.45% and 98.76%, respectively, at an initial concentration of 1000 mg/L. Regression models of metal uptake were developed and validated. A parametric study was done to elucidate the effect of temperature, time and dosage on the biosorption process using both one-factor and two-factor plots. The biosorption of both Zn(II) and Pb(II) was the best fit for Freundlich isotherm and pseudo-second-order kinetic models. The monolayer adsorption capacity of the biosorbent for Zn(II) and Pb(II) was 555.6 mg/g and 909.9 mg/g, respectively. Thermodynamics studies revealed that the adsorption process for both metals was exothermic and spontaneous. It has been shown that the biosorbent from croaker fish scales is an excellent material for the removal of heavy metals from aqueous solutions and consequently for the treatment of industrial effluents.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- AAS:
-
Atomic absorption spectrophotometry
- ANOVA:
-
Analysis of variance
- BBD:
-
Box–Behnken design
- Df:
-
Degree of freedom
- DOE:
-
Design of experiments
- FS:
-
Fish scales
- FTIR:
-
Fourier transform infrared spectroscopy
- Rpm:
-
Rounds per minute
- RSM:
-
Response surface methodology
- SEM:
-
Scanning electron microscopy
- XRD:
-
X-ray diffractometry
- α :
-
Temkin isotherm constant (equilibrium binding constant) (L/mol)
- Ǻ:
-
Armstrong
- b:
-
Temkin constant related to the adsorption heat
- β:
-
Constant related to adsorption energy (mol2/J2)
- C:
-
Constant of the thickness of boundary layer
- C e :
-
Equilibrium concentration (mg/L)
- C i :
-
Initial concentration (mg/L)
- C L :
-
Equilibrium concentration in the liquid phase
- C S :
-
Equilibrium concentration in the solid phase
- K F :
-
Freundlich constant (mg/g)
- K E :
-
Equilibrium constant
- K 1 :
-
Rate constant of the pseudo-first-order model (min−1)
- K 2 :
-
Rate constant of the pseudo-second-order model (gm g−1 min−1)
- K dif :
-
Intra-particle diffusion rate constant (mg/gmin0.5)
- K L :
-
Langmuir constant related to affinity (l/g)
- n :
-
Freundlich constant
- q e :
-
Amount of chemical species removed at equilibrium (mg/g)
- q max :
-
Maximum metal uptake (mg/g)
- q t :
-
Amount of chemical species removed at time t (mg/g)
- R:
-
Universal gas constant 8.314 (J/mol K)
- R 2 :
-
Coefficient of determination
- T :
-
Temperature
- t :
-
Time
- ΔG :
-
Gibbs free energy change
- ΔH :
-
Enthalpy change
- ΔS :
-
Entropy change
References
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Technol 3:74–102
Abdi O, Kazemi M (2015) A review study of biosorption of heavy metals and comparison between different biosorbents. J Mater Environ Sci 6:1386–1399
Abedi S, Zavvar Mousavi H, Asghari A (2015) Investigation of heavy metal ions adsorption by magnetically modified aloe vera leaves ash based on equilibrium, kinetic and thermodynamic studies. Desalin Water Treat 57:13747–13759. https://doi.org/10.1080/19443994.2015.1060536
Adeniyi AG, Ighalo JO (2019) Biosorption of pollutants by plant leaves: an empirical review. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2019.103100
Adeniyi AG, Ighalo JO, Onifade DV (2019) Production of biochar from elephant grass (Pernisetum purpureum) using an updraft biomass gasifier with retort heating. Biofuels. https://doi.org/10.1080/17597269.2018.1554949
Adeniyi AG, Ighalo JO, Onifade DV (2020) Biochar from the thermochemical conversion of orange (Citrus sinensis) peel and Albedo: product quality and potential applications. Chem Afr 3:439–448. https://doi.org/10.1007/s42250-020-00119-6
Afroze S, Kanti T, Ha S, Ang M (2016) Adsorption removal of zinc (II) from aqueous phase by raw and base modified Eucalyptus Sheathiana Bark: kinetics, mechanism and equilibrium study. Process Saf Environ Prot. https://doi.org/10.1016/j.psep.2016.04.009
Ahluwalia SS, Goyal D (2005) Removal of heavy metals by waste tea leaves from aqueous solution. Eng Life Sci 5:158–162
Alluri HK, Ronda SR, Settalluri VS, Bondili JS, Suryanarayana V, Venkateshwar P (2007) Biosorption: an eco-friendly alternative for heavy metal removal. Afr J Biotechnol 6:2924–2931
Amirnia S (2015) Biosorption processes for removal of toxic metals from wastewaters. The University of Western Ontario
Babatunde EO, Akolo SA, Ighalo JO, Kovo AS (2019) Response surface optimisation of the adsorption of Cu(II) from aqueous solution by crab shell chitosan. Paper presented at the 3rd international engineering conference, Minna, Nigeria
Basu A, Mustafiz S, Islam M, Bjorndalen N, Rahaman M, Chaalal O (2006) A comprehensive approach for modeling sorption of lead and cobalt ions through fish scales as an adsorbent. Chem Eng Commun 193:580–605. https://doi.org/10.1080/00986440500193707
Basu A, Rahaman M, Mustafiz S, Islam M (2007) Batch studies of lead adsorption from a multi-component aqueous solution onto Atlantic cod fish scale (Gadus morhua) substrate. J Environ Eng Sci 6:455–462. https://doi.org/10.1139/S06-070
Bhattacharyya KG, Sharma A (2004) Adsorption of Pb(II) from aqueous solution by Azadirachta indica (neem) leaf powder. J Hazard Mater 113:97–109
Chakraborty S, Chowdhury S, Saha PD (2012) Fish (Labeo rohita) scales as a new biosorbent for removal of textile dyes from aqueous solutions. J Water Reuse Desalin 2:175–184. https://doi.org/10.2166/wrd.2012.074
Chandrakanth G, Antía P-G, Ganapati DY, Inmaculada O, Ibanez R, Virendra KR, Kumudini VM (2014) Pharmaceutical industry wastewater: review of the technologies for water treatment and reuse. Ind Eng Chem Res 53:11571–11592
Chowdhury ZZ, Zain SM, Khan RA, Islam MS (2012) Preparation and characterizations of activated carbon from kenaf fiber for equilibrium adsorption studies of copper from wastewater. Korean J Chem Eng 29:1187–1195
Das A, Bhowal A, Datta S (2016) Biomass characterisation and adsorption mechanism of Cu(II) biosorption onto fish (Catla catla) scales. Int J Environ Eng 8:81–94. https://doi.org/10.1504/IJEE.2016.078242
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229
Eletta OAA, Ighalo JO (2019) A review of fish scales as a source of biosorbent for the removal of pollutants from industrial effluents. J Res Inf Civ Eng 16:2479–2510. https://doi.org/10.13140/RG.2.2.20511.61604
Eletta OAA, Adeniyi AG, Magaji MM, Ighalo JO (2019) A mini-review on the application of alumina nano-particles for water treatment. Paper presented at the FUOYE science conference, Oye-ekiti, Nigeria
Eletta OAA, Adeniyi AG, Ighalo JO, Onifade DV, Ayandele FO (2020) Valorisation of Cocoa (Theobroma cacao) pod husk as precursors for the production of adsorbents for water treatment. Environ Technol Rev 9:20–36. https://doi.org/10.1080/21622515.2020.1730983
Goeffrey MG (2008) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol
Huang E (2007) Use of fish scales as biosorbent for the removal of copper in water. Water Res 30:1985–1990
Ighalo JO, Adeniyi AG (2020a) Adsorption of pollutants by plant bark derived adsorbents: an empirical review. J Water Process Eng 35:101228. https://doi.org/10.1016/j.jwpe.2020.101228
Ighalo JO, Adeniyi AG (2020b) A mini-review of the morphological properties of biosorbents derived from plant leaves. SN Appl Sci 2:509. https://doi.org/10.1007/s42452-020-2335-x
Ighalo JO, Adeniyi AG (2020c) Mitigation of Diclofenac pollution in aqueous media by adsorption. ChemBioEng Rev 7:50–64. https://doi.org/10.1002/cben.201900020
Ighalo JO, Adeniyi AG (2020d) Statistical modelling and optimisation of the biosorption of Cd(II) and Pb(II) onto dead biomass of Pseudomonas aeruginosa. Chem Prod Process Model. https://doi.org/10.1515/cppm-2019-0139
Ighalo JO, Eletta AAO (2020) Recent advances in the biosorption of pollutants by fish scales: a mini-review. Chem Eng Commun. https://doi.org/10.1080/00986445.2020.1771322
Iqbal J, Wattoo FH, Wattoo MHS, Malik R, Tirmizi SA, Imran M, Ghangro AB (2011) Adsorption of acid yellow dye on flakes of chitosan prepared from fishery wastes. Arab J Chem 4:389–395. https://doi.org/10.1016/j.arabjc.2010.07.007
Kondapalli S, Mohanty K (2011) Influence of temperature on equilibrium, kinetic and thermodynamic parameters of biosorption of Cr(VI) onto fish scales as suitable biosorbent. J Water Resour Prot 3:429. https://doi.org/10.4236/jwarp.2011.36053
Mandal N, Mondal S, Mondal A, Mukherjee K, Mondal B (2015) Response surface modeling of Cu(II) removal from wastewater using fish scale-derived hydroxyapatite: application of Box–Behnken experimental design. Desalin Water Treat. https://doi.org/10.1080/19443994.2015.1072743
Marrakchi F, Ahmed MJ, Khanday W, Asif M, Hameed B (2016) Mesoporous carbonaceous material from fish scales as low-cost adsorbent for reactive orange 16 adsorption. J Taiwan Inst Chem Eng 71:47–54. https://doi.org/10.1016/j.jtice.2016.12.026
Marrakchi F, Auta M, Khanday W, Hameed B (2017) High-surface-area and nitrogen-rich mesoporous carbon material from fishery waste for effective adsorption of methylene blue. Powder Technol 321:428–434. https://doi.org/10.1016/j.powtec.2017.08.023
Munagapati VS, Yarramuthi V, Nadavala SK, Alla SR, Abburi K (2010) Biosorption of Cu(II), Cd(II) and Pb(II) by Acacia leucocephala bark powder: kinetics, equilibrium and thermodynamics. Chem Eng J 157:357–365
Nadeem R, Ansari TM, Khalid AM (2008) Fourier transform infrared spectroscopic characterization and optimization of Pb(II) biosorption by fish (Labeo rohita) scales. J Hazard Mater 156:64–73. https://doi.org/10.1016/j.jhazmat.2007.11.124
Neves C, Scheufele F, Nardino A, Vieira M, da Silva M, Módenes A, Borba C (2017) Phenomenological modeling of reactive dye adsorption onto fish scales surface in the presence of electrolyte and surfactant mixtures. Environ Technol. https://doi.org/10.1080/09593330.2017.1356876
Othman N, Abd-Kadir A, Zayadi N (2015) Mozambique tilapia fish scales as potential biosorbent for Zn and Pb ions removal: kinetic and isotherm studies. Paper presented at the 2nd IWA Malaysia young water professional conference, Vivatel Hotel, Kuala Lumpur, Malaysia
Othman N, Abd-Kadir A, Zayadi N (2016) Waste fish scale as cost effective adsorbent in removing zinc and ferum ion in wastewater. J Eng Appl Sci 11:1584–1592
Pavia DL, Lampman GM, Kriz GS, Vyvyan JA (2008) Introduction to spectroscopy. Cengage Learning
Prabu K, Shankarlal S, Natarajan E (2012) A biosorption of heavy metal ions from aqueous solutions using fish scale (Catla catla). World J Fish Mar Sci 4:73–77. https://doi.org/10.5829/idosi.wjfms.2012.04.01.56237
Rahaman S, Omi FR, Basu A (2015) Experimental and numerical modelling of arsenic adsorption in fixed-bed dynamic columns packed with Atlantic cod fish scales. Can J Chem Eng 93:2024–2030. https://doi.org/10.1002/cjce.22294
Ramachandra, Ahalya N, Kanamaldi RD (2006) Biosorption: techniques and mechanisms. CES TR 110
Ribeiro C, Scheufele FB, Espinoza-Quinones FR, Módenes AN, da Silva MGC, Vieira MGA, Borba CE (2015) Characterization of Oreochromis niloticus fish scales and assessment of their potential on the adsorption of reactive blue 5G dye. Colloids Surf A Physicochem Eng Asp 482:693–701. https://doi.org/10.1016/j.colsurfa.2015.05.057
Ribeiro C, Scheufele F, Alves H, Kroumov A, Espinoza-Quiñones F, Módenes A, Borba C (2018a) Evaluation of hybrid neutralization/biosorption process for zinc ions removal from automotive battery effluent by dolomite and fish scales. Environ Technol. https://doi.org/10.1080/09593330.2018.1441332
Ribeiro C, Scheufele FB, Espinoza-Quiñones FR, Módenes AN, Vieira MGA, Kroumov AD, Borba CE (2018b) A comprehensive evaluation of heavy metals removal from battery industry wastewaters by applying bio-residue, mineral and commercial adsorbent materials. J Mater Sci 53:7976–7995. https://doi.org/10.1007/s10853-018-2150-6
Srividya K, Mohanty K (2009) Biosorption of hexavalent chromium from aqueous solutions by Catla catla scales: equilibrium and kinetics studies. Chem Eng J 155:666–673. https://doi.org/10.1016/j.cej.2009.08.024
Wen-Kuang L, Bor-Shuang L, Haw-Kai C, Yi-Feng W, Po-Yu C (2017) From waste to health: synthesis of hydroxyapatite scaffolds from fish scales for lead ion removal. JOM 69:713–718. https://doi.org/10.1007/s11837-017-2270-5
WHO (2003) Background document for preparation of WHO Guidelines for drinking-water quality. World Health Organization, Geneva
Zabochnicka-Świątek M, Krzywonos M (2014) Potentials of Biosorption and Bioaccumulation Processes for Heavy Metal Removal. Pol J Environ Stud 23:551–561
Zayadi N, Othman N (2013a) Characterization and optimization of heavy metals biosorption by fish scales. Paper presented at the advanced materials research
Zayadi N, Othman N (2013b) Removal of zinc and ferum ions using tilapia mossambica fish scale. Int J Integr Eng 5:23–29
Zhe H, Hongyuan S, Bicheng H, Chengming L, Yaqin H, Xiaonong C (2014) High-performance fish-scale-based porous carbon for the removal of methylene blue from aqueous solution. RSC Adv 4:18737–18743. https://doi.org/10.1039/b000000x
Zhu K, Gong X, He D, Li B, Ji D, Li P, Peng Z, Luo Y (2013) Adsorption of Ponceau 4R from aqueous solutions using alkali boiled tilapia fish scales. RSC Adv 3:25221–25230. https://doi.org/10.1039/c3ra43817a
Acknowledgements
The authors would love to acknowledge the contributions and guidance of Dr A. G. Adeniyi. Furthermore, the support of Dr G. O. Ighalo. Engr. S. M. Sanusi and Engr. I. A Tijani is acknowledged.
Funding
No research grant or external funding was utilised in this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Human and animal rights
This article does not contain any studies involving human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ighalo, J.O., Eletta, O.A.A. Response surface modelling of the biosorption of Zn(II) and Pb(II) onto Micropogonias undulatus scales: Box–Behnken experimental approach. Appl Water Sci 10, 197 (2020). https://doi.org/10.1007/s13201-020-01283-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-01283-3