Main Challenges and Actions Needed to Improve Conservation and Sustainable Use of Our Crop Wild Relatives
Abstract
:1. Introduction
2. Definition and Classification of CWRs
3. General Threat Status of CWR
4. An Assessment of Peculiarities of CWRs with Respect to Conservation Management
4.1. Biological Peculiarities
4.2. Managerial Responsibility- and Awareness-Related Issues
4.3. The Need for Prioritization of CWR Taxa
- the degree of threat of the species;
- their genetic closeness to the crop species;
- the demand for specific traits/species by the (potential) users (and thus their economic potential);
- the distribution area (uniqueness, incl. endemism; centre of origin/diversity) and occurrence of a given species;
- the conservation status of a given species, including in other (neighboring) countries of the distribution area;
- the (physical as well as legal) availability; and
- the international legal and policy instruments vis-à-vis the national legal framework.
4.4. Availability of and Access to Data and Information
5. The Current CWR Conservation and Use Status
5.1. Facts and Figures on CWR Conservation
5.1.1. In Situ Conservation
5.1.2. Ex Situ Conservation
5.1.3. Complementary Conservation
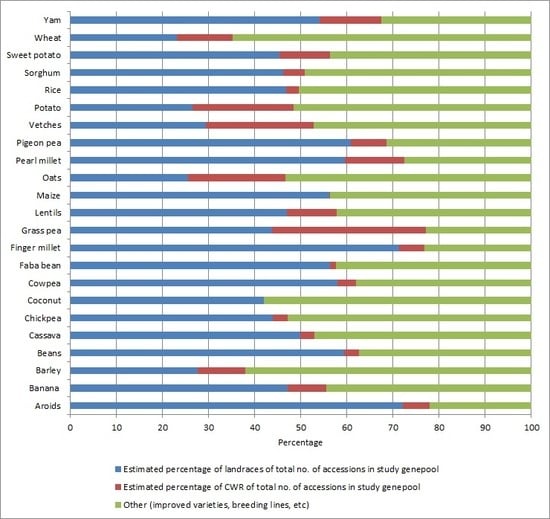
5.2. Facts and Figures on CWR Use
6. What Needs to Be Done to Conserve and Use CWRs More Effectively?
6.1. Documentation
6.2. In Situ Conservation
6.3. Ex Situ Conservation
6.4. Complementary Conservation Approaches
6.5. Supporting Use
6.6. Strengthening the Conservation System
6.6.1. National Level
- Establishment of a comprehensive picture of the national botanic diversity;
- Prioritization of CWR taxa/diversity;
- Eco-geographic and genetic diversity analysis of the priority CWR taxa;
- Identification of threats to priority CWR taxa and important CWR areas;
- Gap analysis and establishment of CWR conservation goals;
- Identification of key national CWR protected areas based on gap analysis, on the CWR inventory and occurrence data, the threat status as well as of CWRs under-represented in genebanks;
- Establishment of national CWR genetic reserves as well as of targeted CWR ex situ collections; and
- Elaboration of concrete suggestions on how to strengthen utilization, research and education.
6.6.2. Local Level
6.6.3. Global Level
7. Conclusions
7.1. Documentation
- Collating, creating, and sharing more information and knowledge on CWR species, in particular, by stimulating and conducting more research.
- Establishing national databases and inventories to enable better coordination and implementation of CWR conservation.
- Developing a global data portal/platform for the exchange and provision of CWR data and information, including tools and guidelines that will facilitate a better coordinated and more efficient conservation, worldwide.
7.2. In Situ Conservation
- Facilitating and encouraging the inclusion of CWRs in national and local conservation agendas and ensuring that they are being given an adequate priority supported possibly by a longer-term financial and organizational structure.
- Complementing the management and monitoring of CWR in situ conservation sites and genetic reserves with ex situ conservation efforts of the priority species.
- Identifying existing and novel mechanisms to finance and govern the proposed global coordination and facilitation of CWR in situ conservation should be of high priority. The proposed global network could play an important role in setting standards, sharing experiences, and providing the platform for monitoring and coordination and, thus, to provide a fundamental basis for ensuring our future food security.
- Increasing the awareness and recognition among actors, especially within the environmental sector, about CWRs as important group of wild species that need to be conserved.
7.3. Ex Situ Conservation
- Ensuring adequate ex situ conservation of threatened national priority CWRs.
- Ensuring adequate ex situ conservation of a globally agreed list of priority CWRs (e.g., [29]) through national/regional/international genebanks, in particular those that already have global or regional conservation responsibilities for the corresponding crop genepools.
- The identification and/or application of new methods to assess the viability of seeds, not requiring seed germination tests, could address current difficulties with viability tests and with small seed samples.
- Large-scale research on CWR seed biology can lead to methods allowing for long-term storage of seeds of these species in genebanks. One such specific research area is the use of cryopreservation for long-term conservation.
7.4. Complementary Conservation and Collaboration
- Development of a generic decision tree on complementary conservation approaches that can be applied to individual CWR species. Supporting guidelines should be developed to facilitate the application of the decision tree and the subsequent implementation of the conservation efforts, using gained experiences with individual species and cases as a basis.
- Ensuring ready access to the genetic resources and related information, both from in situ as well as ex situ conservation within the framework of existing legal instruments.
- Facilitating and coordinating phenotypic and molecular characterization of the priority CWRs to provide a basis for pre-breeding and breeding activities through the involvement of conservation, research, and breeding stakeholders.
- Facilitating/strengthening the collaboration between stakeholders for more effective and efficient conservation, research and use of CWRs as well as to facilitate the transfer of technologies at the local, national, regional, and global levels.
7.5. Conservation System
- Increasing awareness on the importance of and threat to CWRs, including through the active involvement of botanic gardens to ‘demonstrate’ this genetic wealth and the relationship between the CWRs and crop species.
- Facilitating the training of staff on skills that strengthen the implementation of the above activity areas.
- Providing a more stable organizational and financial basis for CWR conservation at national level.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Willcox, G. The beginnings of cereal cultivation and domestication in Southwest Asia. In A Companion to the Archaeology of the Ancient Near East, 1st ed.; Potts, D.T., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2012; pp. 163–180. [Google Scholar]
- Purugganan, M.D. Evolutionary Insights into the Nature of Plant Domestication. Curr. Boil. 2019, 29, R705–R714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Purugganan, M.D.; Fuller, D.Q. The nature of selection during plant domestication. Nature 2009, 457, 843–848. [Google Scholar] [CrossRef] [PubMed]
- White, C.E. The Emergence and Intensification of Cultivation Practices at the Pre-Pottery Neolithic Site of El-Hemmeh, Jordan: An Archaeobotanical Study. Ph.D. Dissertation, Boston University, Boston, MA, USA, 2013; 240p. Available online: https://hdl.handle.net/2144/12888 (accessed on 18 June 2020).
- Weide, A.; Riehl, S.; Zeidi, M.; Conard, N.J. A systematic review of wild grass exploitation in relation to emerging cereal cultivation throughout the Epipalaeolithic and aceramic Neolithic of the Fertile Crescent. PLoS ONE 2018, 13, e0189811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gepts, P. Domestication of Plants. In Encyclopedia of Agriculture and Food Systems; van Alfen, N.K., Ed.; Elsevier: San Diego, CA, USA, 2014; Volume 2, pp. 474–486. [Google Scholar]
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempewolf, H.; Baute, G.; Anderson, J.; Kilian, B.; Smith, C.; Guarino, L. Past and Future Use of Wild Relatives in Crop Breeding. Crop Sci. 2017, 57, 1070–1082. [Google Scholar] [CrossRef]
- Thormann, I.; Engels, J.M.M. Genetic diversity and erosion—A global perspective. In Genetic Diversity and Erosion in Plants—Indicators and Prevention; Ahuja, M.R., Jain, S.M., Eds.; Springer: Berlin, Germany, 2015; Chapter 10; Volume 1, pp. 263–294. [Google Scholar]
- Thormann, I.; Fiorino, E.; Halewood, M.; Engels, J.M.M. Plant genetic resources collections and associated information as baseline resource for genetic diversity studies—An assessment of the IBPGR supported collections. Genet. Resour. Crop Evol. 2015, 62, 1279–1293. [Google Scholar] [CrossRef]
- Rick, C.M.; Chetelat, R. Utilization of related wild species for tomato improvement, First International Symposium on Solanaceae for Fresh Market. Acta Hortic. 1995, 412, 21–38. [Google Scholar] [CrossRef]
- Hoyt, E. Conserving the Wild Relatives of Crops; IBPGR: Rome, Italy, 1988; 45p. [Google Scholar]
- Mammadov, J.; Buyyarapu, R.; Guttikonda, S.K.; Parliament, K.; Abdurakhmonov, I.Y.; Kumpatla, S.P. Wild Relatives of Maize, Rice, Cotton, and Soybean: Treasure Troves for Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2018, 9, 886. [Google Scholar] [CrossRef]
- CBD. Global Strategy for Plant Conservation; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2002; 48p. [Google Scholar]
- CBD. Notification: Strengthening the In Situ Conservation of Plant Genetic Resources for Food and Agriculture through Incorporation of Crop Wild Relatives under Areas Important for Biodiversity in Protected Area Networks and Other Effective Area-Based Conservation Measures (Aichi Biodiversity Targets 7, 11, 12 and 13 and Global Strategy for Plant Conservation Targets 5, 6, 7 and 9); CBD Secretariat: Montreal, QC, Canada, 2015; 11p. [Google Scholar]
- FAO. Second Global Plan of Action for Plant Genetic Resources for Food and Agriculture. Commission on Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; 96p, Available online: http://www.fao.org/3/i2624e/i2624e00.pdf (accessed on 18 June 2020).
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; General Assembly, Seventieth Session; Agenda Items 15 and 116, A/RES/70/1; United Nations: New York, NY, USA, 2015; 35p. [Google Scholar]
- IPBES. Global Assessment Report on Biodiversity and Ecosystem Services; Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services: Bonn, Germany, 2019. [Google Scholar]
- FAO. Voluntary Guidelines for the Conservation and Sustainable Use of Crop Wild Relatives and Wild Food Plants; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; 106p. [Google Scholar]
- Brehm, J.M.; Kell, S.; Thormann, I.; Gaisberger, H.; Dulloo, M.E.; Maxted, N. New tools for crop wild relative conservation planning. Plant Genet. Resour. 2019, 17, 208–212. [Google Scholar] [CrossRef]
- FAO. The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; 399p. [Google Scholar]
- FAO. Assessment of the Implementation of the Second Global Plan of Action for Plant Genetic Resources for Food and Agriculture 2012–2014; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017; 76p. [Google Scholar]
- Maxted, N.; Ford-Lloyd, B.V.; Jury, S.L.; Kell, S.P.; Scholten, M.A. Towards a definition of a crop wild relative. Biodivers. Conserv. 2006, 15, 2673–2685. [Google Scholar] [CrossRef]
- Harlan, J.R.; de Wet, J.M.J. Towards a rational classification of cultivated plants. Taxon 1971, 20, 509–517. [Google Scholar] [CrossRef]
- Maxted, N.; Kell, S.P. Establishment of a Global Network for the In Situ Conservation of Crop Wild Relatives: Status and Needs Background Study Paper No. 39; FAO Commission on Genetic Resources for Food and Agriculture: Rome, Italy, 2009; 224p. [Google Scholar]
- Kell, S.P.; Knüpffer, H.; Jury, S.L.; Ford-Lloyd, B.V.; Maxted, N. Crops and wild relatives of the Euro-Mediterranean region: Making and using a conservation catalogue. In Crop Wild Relative Conservation and Use; Maxted, N., Ford-Lloyd, B.V., Kell, S.P., Iriondo, J.M., Dulloo, M.E., Turok, J., Eds.; CAB International: Wallingford, UK, 2008; pp. 69–109. [Google Scholar]
- Groombridge, B.; Jenkins, M.D. World Atlas of Biodiversity; Prepared by the UNEP World Conservation Monitoring Centre; University of California Press: Berkeley, CA, USA, 2002. [Google Scholar]
- Maxted, N.; Kell, S.P.; Toledo, A.; Dulloo, E.M.; Heywood, V.; Hodgkin, T.; Hunter, D.; Guarino, L.; Jarvis, A.; Ford-Lloyd, B.V. A Global Approach to Crop Wild Relative Conservation: Securing the Gene Pool for Food and Agriculture. Kew Bull. 2010, 65, 561–576. [Google Scholar] [CrossRef]
- Vincent, H.; Wiersema, J.; Dobbie, S.; Kell, S.P.; Fielder, H.; Castañeda-Álvarez, N.P.; Eastwood, R.P.; Guarino, L.; Maxted, N. A prioritized crop wild relative inventory to help underpin global food security. Biol. Conserv. 2013, 167, 265–275. [Google Scholar] [CrossRef]
- The Harlan and de Wet Crop Wild Relatives Inventory. Available online: https://www.cwrdiversity.org/checklist/ (accessed on 30 June 2020).
- Lala, S.; Amri, A.; Maxted, N. Towards the conservation of crop wild relative diversity in North Africa: Checklist, prioritization and inventory. Genet. Resour. Crop Evol. 2018, 65, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Allen, E.; Gaisberger, H.; Brehm, J.M.; Maxted, N.; Thormann, I.; Lupupa, T.; Dulloo, M.E.; Kell, S.P. A crop wild relative inventory for Southern Africa: A first step in linking conservation and use of valuable wild populations for enhancing food security. Plant Genet. Resour. 2019, 17, 128–139. [Google Scholar] [CrossRef]
- CWR Checklists, Strategies, Action Plans. Available online: http://www.cropwildrelatives.org/cwr-strategies/ (accessed on 1 July 2020).
- Ford-Lloyd, B.V.; Schmidt, M.; Armstrong, S.J.; Barazani, O.; Engels, J.; Hadas, R.; Hammer, K.; Kell, S.P.; Kang, D.; Khoshbakht, K.; et al. Crop Wild Relatives—Undervalued, Underutilized and under Threat? BioScience 2011, 61, 559–565. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, A.; Lane, A.; Hijmans, R.J. The Effect of Climate Change on Crop Wild Relatives. Agric. Ecosyst. Environ. 2008, 126, 13–23. [Google Scholar] [CrossRef]
- Lira, R.; Téllez, O.; Dávila, P. The effects of climate change on the geographic distribution of Mexican wild relatives of domesticated Cucurbitaceae. Genet. Resour. Crop Evol. 2009, 56, 691–703. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN Species Survival Commission, IUCN: Gland, Switzerland; Cambridge, UK, 2012; 32p. [Google Scholar]
- Brehm, J.M.; Mitchell, M.; Maxted, N.; Ford-Lloyd, B.V. Martins-Loução, M.A. IUCN Red Listing of Crop Wild Relatives: Is a National Approach as Difficult as Some Think? In Crop Wild Relative Conservation and Use; Maxted, N., Ford-Lloyd, B.V., Kell, S.P., Iriondo, J.M., Dulloo, M.E., Turok, J., Eds.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Ludwig, G.; Haupt, H.; Gruttke, H.; Binot-Hafke, M. Methodik der Gefährdungsanalyse für Rote Listen. In Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Band 1: Wirbeltiere; Haupt, H., Ludwig, G., Gruttke, H., Binot-Hafke, M., Otto, C., Pauly, A., Eds.; Landwirtschaftsverlag: Münster, Germany, 2009; Volume 70, pp. 19–71. [Google Scholar]
- FAO. Study on the Linkages between Protected Areas and the Conservation of Biodiversity for Food and Agriculture. Thematic Study for the State of the World’s Biodiversity for Food and Agriculture; Commission on Genetic Resources for Food and Agriculture, Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; 22p. [Google Scholar]
- Bilz, M.; Kell, S.P.; Maxted, N.; Lansdown, R.V. European Red List of Vascular Plants; Publications Office of the European Union: Brussels, Belgium, 2011; 130p. [Google Scholar]
- Mora, A.; Zapata-Ferrufino, B.; Hunter, D.; Navarro, G.; Galeano, G.; Apaza, K.S.; Baudoin, M.J.; Dulloo, M.E.; Cuellar, S.; Libro Rojo de Parientes Silvestres de Bolivia; et al. Ministerio de Medio Ambiente y Agua, Viceministerio de Medio Ambiente Biodiversidad Y Cambios Climáticos (VMABCC)/Biodiversity International. 2009. Available online: http://www.cropwildrelatives.org/fileadmin/documents/Red%20List_Bolivia_optim.pdf (accessed on 18 June 2020).
- Engels, J.M.M.; (Bioversity International, Rome, Italy). Personal Communication, 2020.
- Rao, K.N.; Dulloo, M.E.; Engels, J.M.M. A review of factors that influence the production of quality seed for long-term conservation in genebanks. Genet. Resour. Crop Evol. 2017, 64, 1061–1074. [Google Scholar]
- Engels, J.M.M. An introduction to plant germplasm exploration and collecting: Planning, methods and procedures, follow-up. In Collecting Plant Genetic Diversity: Technical Guidelines; Guarino, L., Rao, R.V., Goldberg, E., Eds.; Bioversity International: Rome, Italy, 2011. [Google Scholar]
- Hoban, S.; Schlarbaum, S. Optimal sampling of seeds from plant populations for ex-situ conservation of genetic biodiversity, considering realistic population structure. Biol. Conserv. 2014, 177, 90–99. [Google Scholar] [CrossRef]
- Hoban, S.; Strand, A. Ex situ seed collections will benefit from considering spatial sampling design and species’ reproductive biology. Biol. Conserv. 2015, 187, 182–191. [Google Scholar] [CrossRef]
- Bellon, M.R.; Dulloo, E.; Sardos, J.; Thormann, I.; Burdon, J.J. In situ conservation—Harnessing natural and human-derived evolutionary forces to ensure future crop adaptation. Evol. Appl. 2017, 10, 965–977. [Google Scholar] [CrossRef]
- Aguirre-Gutiérrez, J.; van Treuren, R.; Hoekstra, R.; van Hintum, T.J.L. Crop wild relatives range shifts and conservation in Europe under climate change. Divers. Distrib. 2017, 23, 739–750. [Google Scholar] [CrossRef]
- FAO. Genebank Standards for Plant Genetic Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2014; 166p. [Google Scholar]
- Hanson, J.; Ellis, R.H. Progress and Challenges in Ex Situ Conservation of Forage Germplasm: Grasses, Herbaceous Legumes and Fodder Trees. Plants 2020, 9, 446. [Google Scholar] [CrossRef] [Green Version]
- Engels, J.M.M.; Maggioni, L. Managing germplasm in a virtual European genebank (AEGIS) through networking. In Theorien der Lebendsammlung. Pflanzen, Mikroben und Tiere als Biofakte in Genbanken. (Lebenswissenschaften im Dialog 25); Karafyllis, N.C., Ed.; Euro. Verlag: Freiburg/München, Germany, 2018; pp. 169–197. [Google Scholar]
- Ford-Lloyd, B.; Kell, S.P.; Maxted, N. Establishing Conservation Priorities for Crop Wild Relatives. In Crop Wild Relative Conservation and Use; Maxted, N., Ford-Lloyd, B., Kell, S.P., Iriondo, J.M., Dulloo, M.E., Turok, J., Eds.; CAB International: Wallingford, UK, 2008; pp. 110–119. [Google Scholar]
- Akparov, Z.I.; Aronsson, M.; Asdal, A.; Avagyan, A.; Bartha, B.; Benediková, D.; Berishvili, T.; Bocci, R.; Bullinska-Radomska, Z.; Cop, J.; et al. Current and future threats and opportunities facing European crop wild relative and landrace diversity. In Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces; Maxted, N., Dulloo, E.M., Ford-Lloyd, B.V., Frese, L., Iriondo, J., Pinheiro-de-Carvalho, M.A.A., Eds.; CABI: Wallingford, UK, 2011; pp. 333–354. [Google Scholar]
- Hunter, D.; Heywood, V. (Eds.) Crop Wild Relatives. A Manual of in situ Conservation; Routledge: London, UK, 2011; 414p. [Google Scholar]
- Brehm, J.M.; Kell, S.; Thormann, I.; Gaisberger, H.; Dulloo, E.; Maxted, N. Interactive Toolkit for Crop Wild Relative Conservation Planning Version 1.0; University of Birmingham: Birmingham, UK; Bioversity International: Rome, Italy, 2017; Available online: http://www.cropwildrelatives.org/conservation-toolkit/ (accessed on 18 June 2020).
- Thormann, I.; Jarvis, D.I.; Dearing, J.A.; Hodgkin, T. Internationally available information sources for the development of in situ conservation strategies for wild species useful for food and agriculture. Plant Genet. Resour. Newsl. 1999, 118, 38–50. [Google Scholar]
- Thormann, I.; Lane, A.; Durah, K.; Dulloo, M.E.; Gaiji, S. Crop wild relative information: Developing a tool for its management and use. In Crop Wild Relative Conservation and Use; Maxted, N., Ford-Lloyd, B.V., Kell, S.P., Iriondo, J., Dulloo, M.E., Turok, T., Eds.; CAB International: Wallingford, UK, 2008; pp. 504–512. [Google Scholar]
- Thormann, I. Published Sources of Information on Wild Plant Species. In Collecting Plant Genetic Diversity: Technical Guidelines; Guarino, L., Rao, V.R., Goldberg, E., Eds.; Bioversity International: Rome, Italy, 2011; 10p, ISBN 978-92-9043-922-6. Available online: http://cropgenebank.sgrp.cgiar.org/index.php?option=com_content&view=article&id=657 (accessed on 18 June 2020).
- Thormann, I.; Alercia, A.; Dulloo, M.E. Core Descriptors for In Situ Conservation of Crop Wild Relatives v.1; Bioversity International: Rome, Italy, 2013; 28p. [Google Scholar]
- Thormann, I.; Kell, S.; Brehm, J.M.; Dulloo, M.E.; Maxted, N. CWR Checklist and Inventory Data Template v.1; Harvard Dataverse: Cambridge, MA, USA, 2017; Available online: https://dataverse.harvard.edu/dataset.xhtml?persistentId=10.7910/DVN/B8YOQL. (accessed on 18 June 2020). [CrossRef]
- Brehm, J.M.; Kell, S.; Thormann, I.; Gaisberger, H.; Dulloo, E.; Maxted, N. Occurrence Data Collation Template v; Harvard Dataverse: Cambridge, MA, USA, 2017; Available online: https://dataverse.harvard.edu/dataset.xhtml?persistentId=10.7910/DVN/5B9IV5. (accessed on 18 June 2020). [CrossRef]
- Thormann-Wiersema, J.H.; León, B. The GRIN Taxonomy Crop Wild Relative Inventory. In Enhancing Crop Genepool Use: Capturing Wild Relative and Landrace Diversity for Crop Improvement; Maxted, N., Dulloo, M.E., Ford-Lloyd, B.V., Eds.; CAB International: Wallingford, UK, 2016; pp. 453–458. [Google Scholar]
- Bioversity International, University of Birmingham. Crop Wild Relative Checklist and Inventory Descriptors, v.1; Bioversity International: Rome, Italy, 2017; 26p. [Google Scholar]
- Vincent, H.; Amri, A.; Castañeda-Álvarez, N.P.; Dempewolf, H.; Dulloo, E.M.; Guarino, L.; Hole, D.; Mba, C.; Toledo, A.; Maxted, N. Modeling of crop wild relative species identifies areas globally for in situ conservation. Commun. Biol. 2019, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- UNEP-WCMC, IUCN and NGS. Protected Planet Live Report 2020; UNEP-WCMC: Cambridge, UK; IUCN: Gland, Switzerland; NGS: Washington, DC, USA, 2020. [Google Scholar]
- Frese, L.; Bönisch, M.; Herden, T.; Zander, M.; Friesen, N. In-situ-Erhaltung von Wildselleriearten. Nat. Landsch. 2018, 50, 155–163. [Google Scholar]
- Bönisch, M.; Frese, L. Designation of Genetic Reserves for Wild Celery Species in Germany. Crop Wild Relative Issue 12; ISSN 1742-3694 (Online). In Press. Available online: http://farmerspride.eu/.
- Thormann, I. The German Network of Genetic Reserves. Crop Wild Relative Issue 12; ISSN 1742-3694 (Online). In Press. Available online: http://farmerspride.eu/.
- Parra-Quijano, M.; Iriondo, J.M.; Torres, E. Ecogeographical land characterization maps as a tool for assessing plant adaptation and their implications in agrobiodiversity studies. Genet. Resour. Crop Evol. 2011, 59, 205–217. [Google Scholar] [CrossRef]
- Genesys Is an Online Platform Where You Can Find Information about Plant Genetic Resources for Food and Agriculture (PGRFA) Conserved in Genebanks Worldwide. Available online: https://www.genesys-pgr.org/ (accessed on 1 July 2020).
- Eurisco. Finding Seeds for the Future. Available online: http://eurisco.ecpgr.org (accessed on 1 July 2020).
- Castañeda-Álvarez, N.P.; Khoury, C.K.; Achicanoy, H.A.; Bernau, V.; Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Harker, R.H.; Jarvis, A.; Maxted, N.; et al. Global conservation priorities for crop wild relatives. Nat. Plants 2016, 2, 16–22. [Google Scholar] [CrossRef]
- Heywood, V.H. The role of botanic gardens in ex situ conservation of agrobiodiversity. In Implementation of the Global Plan of Action in Europe—Conservation and Sustainable Utilization of Plant Genetic Resources for Food and Agriculture, Proceedings of the European Symposium, Braunschweig, Germany, 30 June–3 July 1988; Gass, T., Frese, L., Begemann, F., Lipman, E., Eds.; International Plant Genetic Resources Institute: Rome, Italy, 1999; pp. 102–107. [Google Scholar]
- Dempewolf, H.; (Global Crop Diversity Trust, Bonn, Germany). Personal communication, 2020.
- Engels, J.; Thormann, I. Final Report of a Consultancy “Increasing Climate Resilience for Poor Farmers: The role of National Plant Genetic Resource Collections”; Kreditanstalt für Wiederaufbau (KfW) and Global Crop Diversity Trust: Frankfurt/Bonn, Germany, 2017; 129p, Unpublished, manuscript in preparation. [Google Scholar]
- Khoury, C.K.; Carver, D.; Kates, H.R.; Achicanoy, H.A.; van Zonneveld, M.; Thomas, E.; Heinitz, C.; Jarret, R.; Labate, J.A.; Reitsma, K.; et al. Distributions, conservation status, and abiotic stress tolerance potential of wild cucurbits (Cucurbita L.). Plants People Planet 2019, 2, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Welcome to PlantSearch! Botanic Gardens Conservation. International. Available online: https://tools.bgci.org/plant_search.php (accessed on 30 June 2020).
- Meyer, A.; Barton, N. Botanic Gardens Are Important Contributors to Crop Wild Relative Preservation. Crop Sci. 2019, 59, 2404–2412. [Google Scholar] [CrossRef] [Green Version]
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Muller, J.V.; Toll, J. Adapting agriculture to climate change: A global initiative to collect, conserve, and use crop wild relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef] [Green Version]
- Engels, J.M.M. Complementary strategies for improved conservation and use of plant genetic resources. In Towards Sustainable National Plant Genetic Resources Programmes—Policy, Planning and Conservation Issues; Engels, J.M.M., Vodouhe, R., Thompson, J., Zannou, A., Hehne, E., Grum, M., Eds.; IPGRI: Rome, Italy, 2000; pp. 69–77. [Google Scholar]
- Hunter, D.; Changtragoon, S. Good practices for conservation and sustainable use of crop wild relatives of tropical fruit tree diversity. In Tropical Fruit Tree Diversity. Good Practices for In Situ and On-Farm Conservation; Sthapit, B., Lamers, H., Rao, R.V., Bailey, A., Eds.; Earthscan from Routledge: London, UK, 2016; pp. 83–96. [Google Scholar]
- Jha, U.C.; Bohra, A.; Singh, N.P. Heat stress in crop plants: Its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed. 2014, 133, 679–701. [Google Scholar] [CrossRef] [Green Version]
- Dulloo, M.E.; Fiorino, E.; Thormann, I. Research on Conservation and Use of Crop Wild Relatives. In Crop Wild Relatives and Climate Change; Chapter 7; Redden, R., Yadav, S.S., Maxted, N., Dulloo, M.E., Guarino, L., Smith, P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 108–129. [Google Scholar]
- Vavilov, N.I. The origin, variation, immunity and breeding of cultivated plants. Chron. Bot. 1951, 13, 1–54. [Google Scholar] [CrossRef]
- Prescott-Allen, C.; Prescott-Allen, R. The First Resource: Wild Species in the North American Economy; Yale University Press: New Haven, CT, USA, 1986. [Google Scholar]
- Engels, J.M.M. Genetische bronnen, hun conservering en aanwending in de aardappelveredeling. In Genetic Resources, Conservation and Use in the Potato Breeding; Agricultural University Wageningen: Wageningen, The Netherlands, 1974; 83p. [Google Scholar]
- Kilian, B.; Martin, W.; Salamini, F. Genetic diversity, evolution and domestication of wheat and barley in the Fertile Crescent. In Evolution in Action; Glaubrecht, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 137–166. [Google Scholar]
- Thormann, I.; Parra-Quijano, M.; Endresen, D.T.F.; Rubio-Teso, M.L.; Iriondo, M.J.; Maxted, N. Predictive Characterization of Crop Wild Relatives and Landraces. Technical Guidelines, Version 1; Bioversity International: Rome, Italy, 2014; 40p. [Google Scholar]
- Willis, K.J. State of the World’s Plants Report—2017; Royal Botanic Gardens: Kew, UK, 2017; 96p. [Google Scholar]
- Bertioli, D.J.; Cannon, S.B.; Froenicke, L.; Huang, G.; Farmer, A.D.; Cannon, E.K.; Liu, X.; Gao, N.; Clevenger, J.; Dash, S.; et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 2016, 48, 438–446. [Google Scholar] [CrossRef]
- PricewaterhouseCoopers PwC, 2013. Crop Wild Relatives: A Valuable Resource for Crop Development. www.pwc.co.uk/valuations. Available online: https://pwc.blogs.com/files/pwc-seed-bank-analysis-for-msb-0713.pdf (accessed on 15 January 2020).
- Pimentel, D.; Wilson, C.; McCullum, C.; Huang, R.; Dwen, P.; Flack, J.; Tran, Q.; Saltman, T.; Cliff, B. Economic and environmental benefits of biodiversity. Bioscience 1997, 47, 747–757. [Google Scholar] [CrossRef]
- Tyack, N.; Dempewolf, H. The Economics of Crop Wild Relatives under Climate Change. In Crop Wild Relatives and Climate Change, 1st ed.; Redden, R., Yadav, S.S., Maxted, N., Dulloo, M.E., Guarino, L., Smith, P., Eds.; Wiley Online Library John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mira, S.; Hill, L.M.; González-Benito, M.E.; Ibáñez, M.A.; Walters, C. Volatile emission in dry seeds as a way to probe chemical reactions during initial asymptomatic deterioration. J. Exp. Bot. 2016, 67, 1783–1793. [Google Scholar] [CrossRef]
- Colville, L.; Bradley, E.L.; Lloyd, A.S.; Pritchard, H.W.; Castle, L.; Kranner, I. Volatile fingerprints of seeds of four species indicate the involvement of alcoholic fermentation, lipid peroxidation, and Maillard reactions in seed deterioration during ageing and desiccation stress. J. Exp. Bot. 2012, 63, 6519–6530. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Plitta-Michalak, B.P.; Naskręt-Barciszewska, M.; Barciszewski, J.; Bujarska-Borkowska, B.; Chmielarz, P. Global 5-methylcytosine alterations in DNA during ageing of Quercus robur seeds. Ann. Bot. 2015, 116, 369–376. [Google Scholar] [CrossRef] [Green Version]
- Mira, S.; Pirredda, M.; Martín-Sánchez, M.; Marchessi, J.; Martín, C. DNA methylation and integrity in aged seeds and regenerated plants. Seed Sci. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Kranner, I.; Chen, H.; Pritchard, H.W.; Pearce, S.R.; Birtic, S. Inter-nucleosomal DNA fragmentation and loss of RNA integrity during seed ageing. Plant Growth Regul. 2011, 63, 63–72. [Google Scholar] [CrossRef]
- Fleming, M.B.; Patterson, E.L.; Reeves, P.A.; Richards, C.M.; Gaines, T.A.; Walters, C. Exploring the fate of mRNA in aging seeds: Protection, destruction, or slow decay? J. Exp. Bot. 2018, 69, 4309–4321. [Google Scholar] [CrossRef]
- Dulloo, M.E.; Ramanatha, V.R.; Engelmann, F.; Engels, J.M.M. Complementary conservation strategy for coconuts. In Coconuts Genetic Resources; Batugal, P., Ramanatha, V.R., Oliver, J., Eds.; International Plant Genetic Resources Institute-Regional Office for Asia, the Pacific, and Oceania (IPGRI-APO): Serdang, Malaysia, 2005; 18p. [Google Scholar]
- Teso, M.L.R.; Torres, M.E.; Parra-Quijano, M.; Iriondo, J.M. Prioritization of crop wild relatives in Spain. Crop Wild Relat. 2012, 18, 18–22. [Google Scholar]
- van Treuren, R. PGR Management in the 21st Century. Crop Wild Relatives: Climate Change and Niche Modeling; Wageningen University and Research, Centre for Genetic Resources: Wageningen, The Netherlands, 2017; Available online: https://edepot.wur.nl/441411 (accessed on 2 May 2020).
- Crop Wild Relatives Global Portal. Available online: http://www.cropwildrelatives.org/ (accessed on 1 July 2020).
- Indigenous and Community Conserved Areas: A Bold New Frontier for Conservation. Available online: https://www.iucn.org/content/indigenous-and-community-conserved-areas-a-bold-new-frontier-conservation (accessed on 1 July 2020).
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Khoury, C.K.; Amariles, D.; Soto, J.S.; Diaz, M.V.; Sotelo, S.; Sosa, C.; Ramírez-Villegas, J.; Achicanoy, H.A.; Velásquez-Tibatá, J.; Guarino, L.; et al. Comprehensiveness of conservation of useful wild plants: An operational indicator for biodiversity and sustainable development targets. Ecol. Indic. 2019, 98, 420–429. [Google Scholar] [CrossRef]
- Dulloo, M.E.; Magos Brehm, J.; Kell, S.; Thormann, I.; Maxted, N. Template for the Preparation of a National Strategic Action Plan for the Conservation and Sustainable Use of Crop Wild Relatives; Harvard Dataverse: Cambridge, MA, USA, 2017; Volume 1, 23p, Available online: https://doi.org/10.7910/DVN/QH9XWB (accessed on 18 June 2020). [CrossRef]
- Brehm, J.M.; Kell, S.; Thormann, I.; Maxted, N.; Dulloo, E. Template for the preparation of a technical background document for a national strategic action plan for the conservation and sustainable use of crop Wild Relatives. Harv. Dataverse 2017, 23. [Google Scholar] [CrossRef]
- Álvarez, N.P.C.; Vincent, H.A.; Kell, S.P.; Eastwood, R.J.; Maxted, N. Ecogeographic surveys. In Collecting Plant Genetic Diversity: Technical Guidelines; Guarino, L., Rao, V.R., Goldberg, E., Eds.; Bioversity International: Rome, Italy, 2011; 23p. [Google Scholar]
- Parra-Quijano, M.; Torres, E.; Iriondo, J.M.; López, F. CAPFITOGEN Tools User Manual, Version 2.0; International Treaty on Plant Genetic Resources for Food and Agriculture, Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; 260p. [Google Scholar]
- Centro Internacional de Agricultura Tropical—CIAT. A Global Database for the Distributions of Crop Wild Relatives. Version 1.12. Available online: https://doi.org/10.15468/jyrthk (accessed on 28 June 2020).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engels, J.M.M.; Thormann, I. Main Challenges and Actions Needed to Improve Conservation and Sustainable Use of Our Crop Wild Relatives. Plants 2020, 9, 968. https://doi.org/10.3390/plants9080968
Engels JMM, Thormann I. Main Challenges and Actions Needed to Improve Conservation and Sustainable Use of Our Crop Wild Relatives. Plants. 2020; 9(8):968. https://doi.org/10.3390/plants9080968
Chicago/Turabian StyleEngels, Johannes M. M., and Imke Thormann. 2020. "Main Challenges and Actions Needed to Improve Conservation and Sustainable Use of Our Crop Wild Relatives" Plants 9, no. 8: 968. https://doi.org/10.3390/plants9080968