Biopharmaceutical Assessment of Dexamethasone Acetate-Based Hydrogels Combining Hydroxypropyl Cyclodextrins and Polysaccharides for Ocular Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gel Composition
2.2.2. Sterilization Step
2.2.3. Physicochemical Characterizations
Drug Quantification
Method Validation
Linearity and Accuracy Studies
Specificity
Precision
Rheological Measurements
2.2.4. Mucoadhesion
2.2.5. Cytotoxicity Studies
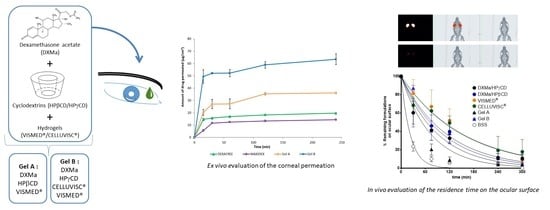
2.2.6. Ex Vivo Evaluation of the Corneal Permeation
2.2.7. In Vivo Evaluation of the Residence Time on the Ocular Surface
2.2.8. Stability Studies
3. Results and Discussion
3.1. Drug Quantification Before and After Sterilization
Method Validation Studies
3.2. Sterilization Step
3.3. Rheological Measurements
Mucoadhesion
3.4. Cytotoxicity Studies
3.4.1. MTT
3.4.2. ALAMAR BLUE® Assay
3.5. Ex Vivo Evaluation of the Corneal Permeation
3.6. In Vivo Evaluation of the Residence Time on the Ocular Surface
3.7. Stability
4. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohira, A.; Hara, K.; Jóhannesson, G.; Tanito, M.; Ásgrímsdóttir, G.M.; Lund, S.H.; Loftsson, T.; Stefánsson, E. Topical dexamethasone γ-cyclodextrin nanoparticle eye drops increase visual acuity and decrease macular thickness in diabetic macular oedema. Act. Ophthalmol. 2015, 93, 610–615. [Google Scholar] [CrossRef]
- Rodríguez Villanueva, J.; Rodríguez Villanueva, L.; Guzmán Navarro, M. Pharmaceutical technology can turn a traditional drug, dexamethasone into a first-line ocular medicine. A global perspective and future trends. Int J. Pharm. 2017, 516, 342–351. [Google Scholar] [CrossRef]
- Pan, Q.; Xu, Q.; Boylan, N.J.; Lamb, N.W.; Emmert, D.G.; Yang, J.-C.; Tang, L.; Heflin, T.; Alwadani, S.; Eberhart, C.G.; et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J. Control. Release 2015, 201, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Hughes, P.M.; Olejnik, O.; Chang-Lin, J.-E.; Wilson, C.G. Topical and systemic drug delivery to the posterior segments. Adv. Drug Deliv. Rev. 2005, 57, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Usayapant, A.; Karara, A.H.; Narurkar, M.M. Effect of 2-hydroxypropyl-beta-cyclodextrin on the ocular absorption of dexamethasone and dexamethasone acetate. Pharm. Res. 1991, 8, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, H.M.; Kupferman, A.; Stewart, R.H.; Kimbrough, R.L. Evaluation of dexamethasone acetate as a topical ophthalmic formulation. Am. J. Ophthalmol. 1978, 86, 418–423. [Google Scholar] [CrossRef]
- Mazet, R.; Choisnard, L.; Levilly, D.; Wouessidjewe, D.; Gèze, A. Investigation of combined cyclodextrin and hydrogel formulation for ocular delivery of dexamethasone acetate by means of experimental designs. Pharmaceutics 2018, 10, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillon, M.; Maissa, C.; Ho, S. Evaluation of the effects on conjunctival tissues of optive eyedrops over one month usage. Cont. Lens Anterior Eye 2010, 33, 93–99. [Google Scholar] [CrossRef]
- Chatterjee, B.; Amalina, N.; Sengupta, P.; Kumar Mandal, U. Mucoadhesive polymers and their mode of action: A recent update. J. Appl. Pharm. Sci. 2017, 7, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Oh, H.J.; Li, Z.; Park, S.-H.; Yoon, K.C. Effect of hypotonic 0.18% sodium hyaluronate eyedrops on inflammation of the ocular surface in experimental dry eye. J. Ocul. Pharm. Ther. 2014, 30, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.C.C.; Mainardes, R.M.; Gremião, M.P.D. Development and validation of HPLC method for analysis of dexamethasone acetate in microemulsions. Braz. J. Pharm. Sci. 2009, 45, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Borman, P.; Elder, D. Q2(R1) Validation of Analytical Procedures. In ICH Quality Guidelines; Teasdale, A., Elder, D., Nims, R.W., Eds.; ICH: Geneva, Switzerland, 2017. [Google Scholar] [CrossRef]
- Space, J.S.; Opio, A.M.; Nickerson, B.; Jiang, H.; Dumont, M.; Berry, M. Validation of a dissolution method with HPLC analysis for lasofoxifene tartrate low dose tablets. J. Pharm. Biomed. Anal. 2007, 44, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Sautou, V.; Bossard, D.; Chedru-Legros, V.; Crauste-Manciet, S.; Fleury-Souverain, S.; Lagarce, F.; Odou, P.; Roy, S.; Sadeghipour, F.; Sautou, V. Methodological Guidelines for Stability Studies of Hospital Pharmaceutical Preparations, 1st ed.; GERPAC, SFPC, Eds.; 2013; p. 75. Available online: https://www.gerpac.eu/IMG/pdf/guide_stabilite_anglais.pdf (accessed on 20 February 2018).
- Graça, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Useful In vitro techniques to evaluate the mucoadhesive properties of hyaluronic acid-based ocular delivery systems. Pharmaceutics 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [Green Version]
- da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef]
- Rönkkö, S.; Vellonen, K.-S.; Järvinen, K.; Toropainen, E.; Urtti, A. Human corneal cell culture models for drug toxicity studies. Drug Deliv. Transl. Res. 2016, 6, 660–675. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Ban, J.; Mo, Z.; Zhang, Y.; An, P.; Liu, L.; Xie, Q.; Du, Y.; Xie, B.; Zhan, X.; et al. A potential nanoparticle-loaded in situ gel for enhanced and sustained ophthalmic delivery of dexamethasone. Nanotechnology 2018, 29, 425101. [Google Scholar] [CrossRef]
- National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The national academies collection: Reports funded by national institutes of health; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Fernández-Ferreiro, A.; Silva-Rodríguez, J.; Otero-Espinar, F.J.; González-Barcia, M.; Lamas, M.J.; Ruibal, A.; Luaces-Rodriguez, A.; Vieites-Prado, A.; Sobrino, T.; Herranz, M.; et al. Positron emission tomography for the development and characterization of corneal permanence of ophthalmic pharmaceutical formulations. Invest. Ophthalmol. Vis. Sci. 2017, 58, 772–780. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef]
- European Phramacopoeia, 9.8th ed.; EDQM: Strasbourg, France, 2019.
- McEvoy, G.K. American Society of Health-System Pharmacists. AHFS Drug information 2007; American Society of Health-System Pharmacists: Bethesda, MD, USA, 2007; ISBN 978-1-58528-161-9. [Google Scholar]
- Zaki, I.; Fitzgerald, P.; Hardy, J.G.; Wilson, C.G. A comparison of the effect of viscosity on the precorneal residence of solutions in rabbit and man. J. Pharm. Pharmcol. 1986, 38, 463–466. [Google Scholar] [CrossRef]
- Snibson, G.R.; Greaves, J.L.; Soper, N.D.; Prydal, J.I.; Wilson, C.G.; Bron, A.J. Precorneal residence times of sodium hyaluronate solutions studied by quantitative gamma scintigraphy. Eye 1990, 4, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; D′Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of hyaluronan-based eye drop formulations. Carbohydr. Polym. 2016, 153, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franck, A. Understanding Rheology of Structured Fluids; TA Instruments: New Castle, DE, USA, 2004; pp. 1–11. Available online: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Understanding+Rheology+of+Structured+Fluids#2%5Cnhttp://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle (accessed on 27 March 2018).
- Madsen, F.; Eberth, K.; Smart, J.D. A rheological examination of the mucoadhesive/mucus interaction: The effect of mucoadhesive type and concentration. J. Control. Release 1998, 50, 167–178. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- OECD. Guideline for the Testing of Chemicals—Short Time Exposure in Vitro Test Method; Organisation for Economic Co-operation and Development: Paris, France, 2018; p. 19. Available online: https://www.oecd-ilibrary.org/environment/test-no-491-short-time-exposure-in-vitro-test-method-for-identifying-i-chemicals-inducing-serious-eye-damage-and-ii-chemicals-not-requiring-classification-for-eye-irritation-or-serious-eye-damage_9789264242432-en (accessed on 27 March 2018).
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Study of the relationship between lipid binding properties of cyclodextrins and their effect on the integrity of liposomes. Int. J. Pharm. 2007, 338, 35–42. [Google Scholar] [CrossRef]
- Fernández-Ferreiro, A.; Fernández Bargiela, N.; Varela, M.S.; Martínez, M.G.; Pardo, M.; Piñeiro Ces, A.; Méndez, J.B.; Barcia, M.G.; Lamas, M.J.; Otero-Espinar, F. Cyclodextrin–polysaccharide-based, in situ-gelled system for ocular antifungal delivery. Beilstein J. Org. Chem. 2014, 10, 2903–2911. [Google Scholar] [CrossRef] [Green Version]
- Zoorob, R.J.; Cender, D. A different look at corticosteroids. Am. Fam. Physician 1998, 58, 443–450. [Google Scholar]
- Behl, G.; Iqbal, J.; O′Reilly, N.J.; McLoughlin, P.; Fitzhenry, L. Synthesis and characterization of poly(2-hydroxyethylmethacrylate) contact lenses containing chitosan nanoparticles as an ocular delivery system for dexamethasone sodium phosphate. Pharm. Res. 2016, 33, 1638–1648. [Google Scholar] [CrossRef]
- Djalilian, A.R.; Nagineni, C.N.; Mahesh, S.P.; Smith, J.A.; Nussenblatt, R.B.; Hooks, J.J. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporin. Cornea 2006, 25, 709–714. [Google Scholar] [CrossRef]
- Castro-Balado, A.; Mondelo-García, C.; González-Barcia, M.; Zarra-Ferro, I.; Otero-Espinar, F.J.; Ruibal-Morell, Á.; Aguiar, P.; Fernández-Ferreiro, A. Ocular biodistribution studies using molecular imaging. Pharmaceutics 2019, 11, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luaces-Rodríguez, A.; Touriño-Peralba, R.; Alonso-Rodríguez, I.; García-Otero, X.; González-Barcia, M.; Rodríguez-Ares, M.T.; Martínez-Pérez, L.; Aguiar, P.; Gómez-Lado, N.; Silva-Rodríguez, J.; et al. Preclinical characterization and clinical evaluation of tacrolimus eye drops. Eur. J. Pharm. Sci. 2018, 120, 152–161. [Google Scholar] [CrossRef] [PubMed]
- ICH. Stability testing of new drug substances and products Q1A (R2). In Proceedings of the International Conference on Harmonisation; ICH: Geneva, Switzerland, 2003; pp. 1–20. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 27 March 2018).










| Mixed Gels | Components | Quantity (g) |
|---|---|---|
| Optimized mixed Gel A | VISMED® | 0.300 |
| HPβCD 600 mg/mL with DXMa | 0.700 | |
| Optimized mixed Gel A contains 7 mg/g of DXMa and an osmolality of 449 mOsm/kg | ||
| Optimized mixed Gel B | CELLUVISC® | 0.151 |
| VISMED® | 0.085 | |
| HPγCD 600 mg/mL with DXMa | 0.764 | |
| Optimized mixed Gel B contains 20 mg/g of DXMa and an osmolality of 425 mOsm/kg | ||
| Gels | Level 80% | Level 90% | Level 100% | Level 110% | Level 120% |
|---|---|---|---|---|---|
| Gel A (µg/mL) | 56 | 63 | 70 | 77 | 84 |
| Gel B (µg/mL) | 160 | 180 | 200 | 220 | 240 |
| Cell Viability | UN GHS Classification | Applicability | DXMa Formulation Vehicles | |
|---|---|---|---|---|
| At 5% | At 0.05% | |||
| >70% | >70% | No category | No serious damage nor eye irritation effect | Gel B |
| HPγCD aqueous solution (600 mg/mL) | ||||
| ≤70% | >70% | No prediction can be made | No prediction can be made, eventual eye irritation | Gel A |
| HPβCD aqueous solution (600 mg/mL) | ||||
| ≤70% | ≤70% | Category 1 | Serious eye damage | None |
| Components | Viscosity at 35 °C (mPa.s) | k (min−1) | T1/2 (min) | MRT (min) | R2 |
|---|---|---|---|---|---|
| CELLUVISC® | 167–260 | 0.007 ± 0.003 | 136.5 ± 95.5 | 196.9 ± 137.8 | 0.9738 |
| VISMED® | 16.8 | 0.008 ± 0.003 | 92.7 ± 26.7 | 133.7 ± 38.5 | 0.9404 |
| Gel B | 18.6 | 0.0096 ± 0.036 | 77.4 ± 28.8 | 111.6 ± 41.5 | 0.9837 |
| Gel A | 13.2 | 0.015 ± 0.002 | 46.6 ± 4.8 | 67.2 ± 6.9 | 0.9365 |
| DXMa/HPβCD | 6.4 | 0.015 ± 0.014 | 81.7± 59.0 | 117.9± 85.2 | 0.9866 |
| (10 mg/mL/600 mg/mL) | |||||
| DXMa/HPγCD | 6.5 | 0.11 ± 0.003 | 70.2 ± 21.9 | 101.3 ± 31.6 | 0.9697 |
| (30 mg/mL/600 mg/mL) | |||||
| BSS | 1.5 | 0.046 ± 0.015 | 16.0 ± 5.2 | 23.1 ± 7.6 | 0.9965 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazet, R.; García-Otero, X.; Choisnard, L.; Wouessidjewe, D.; Verdoot, V.; Bossard, F.; Díaz-Tomé, V.; Blanc-Marquis, V.; Otero-Espinar, F.-J.; Fernandez-Ferreiro, A.; et al. Biopharmaceutical Assessment of Dexamethasone Acetate-Based Hydrogels Combining Hydroxypropyl Cyclodextrins and Polysaccharides for Ocular Delivery. Pharmaceutics 2020, 12, 717. https://doi.org/10.3390/pharmaceutics12080717
Mazet R, García-Otero X, Choisnard L, Wouessidjewe D, Verdoot V, Bossard F, Díaz-Tomé V, Blanc-Marquis V, Otero-Espinar F-J, Fernandez-Ferreiro A, et al. Biopharmaceutical Assessment of Dexamethasone Acetate-Based Hydrogels Combining Hydroxypropyl Cyclodextrins and Polysaccharides for Ocular Delivery. Pharmaceutics. 2020; 12(8):717. https://doi.org/10.3390/pharmaceutics12080717
Chicago/Turabian StyleMazet, Roseline, Xurxo García-Otero, Luc Choisnard, Denis Wouessidjewe, Vincent Verdoot, Frédéric Bossard, Victoria Díaz-Tomé, Véronique Blanc-Marquis, Francisco-Javier Otero-Espinar, Anxo Fernandez-Ferreiro, and et al. 2020. "Biopharmaceutical Assessment of Dexamethasone Acetate-Based Hydrogels Combining Hydroxypropyl Cyclodextrins and Polysaccharides for Ocular Delivery" Pharmaceutics 12, no. 8: 717. https://doi.org/10.3390/pharmaceutics12080717