Abstract
Purpose
The active ingredient recovery from the vegetable is a very attractive research field for the development of a sustainable economy; to revalue the jujube fruit (Zizyphus lotus) polysaccharide (ZLPS), an optimized green microwave-assisted method was used for the recovery and enrichment of the antioxidants present in a distilled water extract.
Methods
A series of 17 experiments including microwave power, irradiation time, and liquid-to-solid ratio independent parameters was designed by the response surface methodology to optimize the recovery of the polysaccharide extract.
Results
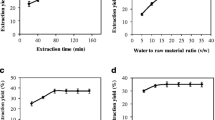
The optimal conditions were as follows: 600 W, 40 min, and 26.69 mL/g. Under these conditions, the experimental extraction yield was 13.98 ± 1.55% which is very close with the predicted value (14.08%), and this demonstrated the validation of the extraction model proposed. The polysaccharide extract exhibited a significant scavenging activity against ABTS.+ (70.45%), DPPH*.(66.02%), and FRAP (A = 0.63) with a very important anti-inflammatory activity using a protein denaturation method that showed a maximum inhibition of 95.33% at 200 μg/mL. Additionally, the membrane stabilization method showed a significant action and protection of human red blood cells (85.76%) in hypotonic-induced lysis solution and 86.45% in heat-induced lysis solution.
Conclusion
This study demonstrated the possibility of exploiting the microwave process to obtain extracts remarkably enriched with invaluable antioxidants from the jujube matrix. The operation time is short, and the antioxidant and anti-inflammatory activities of the distilled water extract were preserved.




Similar content being viewed by others
References
Hammi KM, Jdey A, Abdelly C, Majdoub H, Ksouri R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015;184:80–9. https://doi.org/10.1016/j.foodchem.2015.03.047.
Abdeddaim M, et al. Biochemical characterization and nutritional properties of Zizyphus lotus l. fruits in aures region, northeastern of Algeria. Ann Food Sci Technol. 2014;15(1):75–81.
Samavati V, Manoochehrizade A. Polysaccharide extraction from Malva sylvestris and its anti-oxidant activity. Int J Biol Macromol. 2013;60:427–36. https://doi.org/10.1016/j.ijbiomac.2013.04.050.
Abbou A, Kadri N, Debbache N, Dairi S, Remini H, Dahmoune F, et al. Effect of precipitation solvent on some biological activities of polysaccharides from Pinus halepensis Mill. seeds. Int J Biol Macromol. 2019;141:663–70. https://doi.org/10.1016/j.ijbiomac.2019.08.266.
Zhao Z, Li J, Wu X, Dai H, Gao X, Liu M, et al. Structures and immunological activities of two pectic polysaccharides from the fruits of Ziziphus jujuba Mill. cv. jinsixiaozao Hort. Food Res Int. 2006;39(8):917–23. https://doi.org/10.1016/j.foodres.2006.05.006.
Wang J, Hu S, Nie S, Yu Q, Xie M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Med Cell Longev. 2016;2016:1–13. https://doi.org/10.1155/2016/5692852.
Hammi KM, et al. Optimization extraction of polysaccharide from Tunisian Zizyphus lotus fruit by response surface methodology: composition and antioxidant activity. Food Chem. 2016;212:476–84. https://doi.org/10.1016/j.foodchem.2016.06.004.
Thirugnanasambandham K, Sivakumar V, Maran JP. Microwave-assisted extraction of polysaccharides from mulberry leaves. Int J Biol Macromol. 2015;72:1–5. https://doi.org/10.1016/j.ijbiomac.2014.07.031.
Dahmoune F, Nayak B, Moussi K, Remini H, Madani K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015;166:585–95. https://doi.org/10.1016/j.foodchem.2014.06.066.
Dahmoune F, Spigno G, Moussi K, Remini H, Cherbal A, Madani K. Pistacia lentiscus leaves as a source of phenolic compounds: microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind Crop Prod. 2014;61:31–40. https://doi.org/10.1016/j.indcrop.2014.06.035.
Abbou A, Kadri N, Dahmoune F, Chergui A, Remini H, Berkani F, et al. Optimising functional properties and chemical composition of Pinus halepensis Mill. Seeds protein concentrates. Food Hydrocoll. 2020;100:105416. https://doi.org/10.1016/j.foodhyd.2019.105416.
Yuan Y, Liu Y, Liu M, Chen Q, Jiao Y, Liu Y, et al. Optimization extraction and bioactivities of polysaccharide from wild Russula griseocarnosa. Saudi Pharm J. 2017;25(4):523–30. https://doi.org/10.1016/j.jsps.2017.04.018.
Rostami H, Gharibzahedi SMT. Microwave-assisted extraction of jujube polysaccharide: optimization, purification and functional characterization. Carbohydr Polym. 2016;143:100–7. https://doi.org/10.1016/j.carbpol.2016.01.075.
Chen Y, Xie M, Li W, Zhang H, Nie S, Wang Y, et al. An effective method for deproteinization of bioactive polysaccharides extracted from lingzhi (Ganoderma atrum). Food Sci Biotechnol. 2012;21(1):191–8. https://doi.org/10.1007/s10068-012-0024-2.
Singh, B., R. Kumar, and N. Ahuja, Optimizing drug delivery systems using systematic" design of experiments." Part I: fundamental aspects. Critical Reviews™ in Therapeutic Drug Carrier Systems, 2005. 22(1). doi: https://doi.org/10.1615/CritRevTherDrugCarrierSyst.v22.i1.20
Dahmoune, F., et al., Ultrasound assisted extraction of phenolic compounds from P. lentiscus L. leaves: comparative study of artificial neural network (ANN) versus degree of experiment for prediction ability of phenolic compounds recovery. 2015. 77: p. 251–261. https://doi.org/10.1016/j.indcrop.2015.08.062
Dubois M, et al. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350–6. https://doi.org/10.1021/ac60111a017.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54. https://doi.org/10.1016/0003-2697(76)90527-3.
Achat S, Tomao V, Madani K, Chibane M, Elmaataoui M, Dangles O, et al. Direct enrichment of olive oil in oleuropein by ultrasound-assisted maceration at laboratory and pilot plant scale. Ultrason Sonochem. 2012;19(4):777–86. https://doi.org/10.1016/j.ultsonch.2011.12.006.
Long LH, Kwee DCT, Halliwell B. The antioxidant activities of seasonings used in Asian cooking. Powerful antioxidant activity of dark soy sauce revealed using the ABTS assay. Free Radic Res. 2000;32(2):181–6. https://doi.org/10.1080/10715760000300181.
Karthik, I., Evaluation of anti-inflammatory activity of Canthium parviflorum by in-vitro method. 2013.
Rani AA, Punitha SMJ, Rema M. Anti-inflammatory activity of flower extract of Cassia auriculata –an in-vitro study. Int Res J Pharm Appl Sci. 2014;4:57–60.
Abd-Alrahman SH, et al. Phytochemical screening and antimicrobial activity of EthOH/water Ziziphus jujuba seeds extracts. J Pure Appl Microbiol. 2013;7:823–8.
Umapathy E, et al. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J Med Plant Res. 2010;4(9):789–95. https://doi.org/10.5897/JMPR10.056.
Oyedapo O, et al. Red blood cell membrane stabilizing potentials of extracts of Lantana camara and its fractions. Int J Plant Physiol Biochem. 2010;2(4):46–51. https://doi.org/10.5897/IJPPB.
Zhou C, Yu X, Ma H, Liu S, Qin X, Yagoub AEGA, et al. Examining of athermal effects in microwave-induced glucose/glycine reaction and degradation of polysaccharide from Porphyra yezoensis. Carbohydr Polym. 2013;97(1):38–44. https://doi.org/10.1016/j.carbpol.2013.04.033.
Adeli M, Samavati V. Studies on the steady shear flow behavior and chemical properties of water-soluble polysaccharide from Ziziphus lotus fruit. Int J Biol Macromol. 2015;72:580–7. https://doi.org/10.1016/j.ijbiomac.2014.08.047.
Hayat K, Hussain S, Abbas S, Farooq U, Ding B, Xia S, et al. Optimized microwave-assisted extraction of phenolic acids from citrus mandarin peels and evaluation of antioxidant activity in vitro. Sep Purif Technol. 2009;70(1):63–70. https://doi.org/10.1016/j.seppur.2009.08.012.
Liu J, Liu H, Ma L, Wang S, Gao J, Li Y, et al. A Chinese jujube (Ziziphus jujuba Mill.) fruit-expressed sequence tag (EST) library: annotation and EST-SSR characterization. Sci Hortic. 2014;165:99–105. https://doi.org/10.1016/j.scienta.2013.10.033.
Chouaibi, M., Mahfoudhi N., Rezig L., Donsi F., Ferrari G., Hamdi S., A comparative study on physicochemical, rheological and surface tension properties of Tunisian jujube (Zizyphus lotus L.) seed and vegetable oils. Int J Food Eng, 2012. 8(2). https://doi.org/10.1515/1556-3758.2759
Chouaibi M, Rezig L, Ben daoued K, Mahfoudhi N, Bouhafa H, Hamdi S. Extraction of polysaccharide from zizyphus lotus fruits. Int J Food Eng. 2012;8(3). https://doi.org/10.1515/1556-3758.2756.
Kamnev AA, Colina M, Rodriguez J, Ptitchkina NM, Ignatov VV. Comparative spectroscopic characterization of different pectins and their sources. Food Hydrocoll. 1998;12(3):263–71. https://doi.org/10.1016/S0268-005X(98)00014-9.
Coimbra MA, Gonçalves F, Barros AS, Delgadillo I. Fourier transform infrared spectroscopy and chemometric analysis of white wine polysaccharide extracts. J Agric Food Chem. 2002;50(12):3405–11. https://doi.org/10.1021/jf020074p.
Sun Y-D, Wang Z-H, Ye Q-S. Composition analysis and anti-proliferation activity of polysaccharides from Dendrobium chrysotoxum. Int J Biol Macromol. 2013;62:291–5. https://doi.org/10.1016/j.ijbiomac.2013.08.046.
Xu Y, Cai F, Yu Z, Zhang L, Li X, Yang Y, et al. Optimisation of pressurised water extraction of polysaccharides from blackcurrant and its antioxidant activity. Food Chem. 2016;194:650–8. https://doi.org/10.1016/j.foodchem.2015.08.061.
Wu Z, Li H, Tu D, Yang Y, Zhan Y. Extraction optimization, preliminary characterization, and in vitro antioxidant activities of crude polysaccharides from finger citron. Ind Crop Prod. 2013;44:145–51. https://doi.org/10.1016/j.indcrop.2012.11.008.
Li J, Liu Y, Fan L, Ai L, Shan L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohydr Polym. 2011;84(1):390–4. https://doi.org/10.1016/j.carbpol.2010.11.051.
Lin T, Liu Y, Lai C, Yang T, Xie J, Zhang Y. The effect of ultrasound assisted extraction on structural composition, antioxidant activity and immunoregulation of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds. Ind Crop Prod. 2018;125:150–9. https://doi.org/10.1016/j.indcrop.2018.08.078.
Ferreira IC, et al. Free-radical scavenging capacity and reducing power of wild edible mushrooms from Northeast Portugal: individual cap and stipe activity. Food Chem. 2007;100(4):1511–6. https://doi.org/10.1016/j.foodchem.2005.11.043.
Ananthi S, et al. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (Marine Brown Alga). Food Chem Toxicol. 2010;48(1):187–92. https://doi.org/10.1016/j.fct.2009.09.036.
Oyedapo O, Famurewa A. Antiprotease and membrane stabilizing activities of extracts of Fagara zanthoxyloides, Olax subscorpioides and Tetrapleura tetraptera. Int J Pharmacogn. 1995;33(1):65–9. https://doi.org/10.3109/13880209509088150.
Sonibare MA, Onda EE, Ajayi AM, Umukoro S. In vitro antioxidant and membrane stabilization activities of the fruit extract and fractions of Tetrapleura tetraptera (Schumach & Thonn.) Taub. J Pharm Biores. 2015;12(2):95–105. https://doi.org/10.4314/jpb.v12i2.3.
Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol. 1968;20(3):169–73. https://doi.org/10.1111/j.2042-7158.1968.tb09718.x.
Mzoughi Z, Abdelhamid A, Rihouey C, le Cerf D, Bouraoui A, Majdoub H. Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohydr Polym. 2018;185:127–37. https://doi.org/10.1016/j.carbpol.2018.01.022.
Wu Y, Cui SW, Tang J, Wang Q, Gu X. Preparation, partial characterization and bioactivity of water-soluble polysaccharides from boat-fruited sterculia seeds. Carbohydr Polym. 2007;70(4):437–43. https://doi.org/10.1016/j.carbpol.2007.05.010.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berkani, F., Dahmoune, F., Achat, S. et al. Response Surface Methodology Optimization of Microwave-Assisted Polysaccharide Extraction from Algerian Jujube (Zizyphus lotus L.) Pulp and Peel. J Pharm Innov 16, 630–642 (2021). https://doi.org/10.1007/s12247-020-09475-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-020-09475-9