Abstract
Background
Interlamellar bonding in the arterial wall is often compromised by cardiovascular diseases. However, several recent nationwide and hospital-based studies have uniformly reported reduced risk of thoracic aortic dissection in patients with diabetes. As one of the primary structural constituents in the arterial wall, elastin plays an important role in providing its interlamellar structural integrity.
Objective
The purpose of this study is to examine the effects of glycation on the interlamellar bonding properties of arterial elastin.
Methods
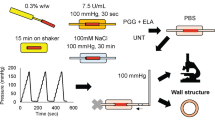
Purified elastin network was isolated from porcine descending thoracic aorta and incubated in 2 M glucose solution for 7, 14 or 21 days at 37 °C. Peeling and direct tension tests were performed to provide complimentary information on understanding the interlamellar layer separation properties of elastin network with glycation effect. Peeling tests were simulated using a cohesive zone model (CZM). Multiphoton imaging was used to visualize the interlamellar elastin fibers in samples subjected to peeling and direct tension.
Results
Peeling and direct tension tests show that interlamellar energy release rate and strength both increase with the duration of glucose treatment. The traction at damage initiation estimated for the CZM agrees well with the interlamellar strength measurements from direct tension tests. Glycation was also found to increase the interlamellar failure strain of arterial elastin. Multiphoton imaging confirmed the contribution of radially running elastin fibers to resisting dissection.
Conclusions
Nonenzymatic glycation reduces the propensity of arterial elastin to dissection. This study also suggests that the CZM effectively describes the interlamellar bonding properties of arterial elastin.









Similar content being viewed by others
References
Kielty CM, Sherratt MJ, Shuttleworth CA (2002) Elastic fibres. J Cell Sci 155:2817–2828
Avolio A, Jones D, Tafazzoli-Shadpour M (1998) Quantification of alterations in structure and function of elastin in the arterial media. Hypertension 32:170–175
Clark TE, Lillie MA, Vogl AW, Gosline JM, Shadwick RE (2015) Mechanical contribution of lamellar and interlamellar elastin along the mouse aorta. J Biomech 48:3599–3605
Noble C, Smulders N, Lewis R, Carré MJ, Franklin SE, MacNeil S, Taylor ZA (2016) Controlled peel testing of a model tissue for diseased aorta. J Biomech 49:3667–3675
Pasta S, Phillippi JA, Gleason TG, Vorp DA (2012) Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg 143:460–467
Wang R, Yu X, Zhang Y (2020) Mechanical and structural contributions of elastin and collagen fibers to interlamellar bonding in the arterial wall. Biomech Model Mechanobiol. https://doi.org/10.1007/s10237-020-01370-z
Rosenbloom J, Abrams WR, Mecham R (1993) Extracellular matrix 4: the elastic fiber. FASEB J 7:1208–1218
Wolinsky H, Glagov S (1964) Structural basis for the static mechanical properties of the aortic media. Circ Res 14:400–413
Wolinsky H, Glagov S (1967) A lamellar unit of aortic medial structure and function in mammals. Circ Res 20:99–111
O’Connell MK, Murthy S, Phan S, Xu C, Buchanan JA, Spilker R, Dalman RL, Zarins CK, Denk W, Taylor CA (2008) The three-dimensional micro- and nanostructure of the aortic medial lamellar unit measured using 3D confocal and electron microscopy imaging. Matrix Biol 27:171–181
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, Fattori R, Suzuki T, Oh JK, Moore AG, Malouf JF, Pape LA, Gaca C, Sechtem U, Lenferink S, Deutsch HJ, Diedrichs H, Marcos y Robles J, Llovet A, Gilon D, Das SK, Armstrong WF, Deeb GM, Eagle KA (2000) The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA 283:897–903
Khan IA, Nair CK (2002) Clinical, diagnostic, and management perspectives of aortic dissection. Chest 122:311–328
Spittell PC, Spittell JA, Joyce JW, Tajik AJ, Edwards WD, Schaff HV, Stanson AW (1993) Clinical features and differential diagnosis of aortic dissection: experience with 236 cases (1980 through 1990). Mayo Clin Proc 68:642–651
Thubrikar MJ, Agali P, Robicsek F (1999) Wall stress as a possible mechanism for the development of transverse intimal tears in aortic dissections. J Med Eng Technol 23:127–134
Carson MW, Roach MR (1990) The strength of the aortic media and its role in the propagation of aortic dissection. J Biomech 23:579–588
Tam ASM, Sapp MC, Roach MR (1998) The effect of tear depth on the propagation of aortic dissections in isolated porcine thoracic aorta. J Biomech 31:673–676
Sommer G, Gasser TC, Auer M, Regitnig P, Holzapfel GA (2008) Dissection properties of the human aortic media: an experimental study. J Biomech Eng 130:021007
Tong J, Cohnert T, Regitnig P, Kohlbacher J, Birner-Gruenberger R, Schriefl AJ, Sommer G, Holzapfel GA (2014) Variations of dissection properties and mass fractions with thrombus age in human abdominal aortic aneurysms. J Biomech 47:14–23
Tong J, Sommer G, Regitnig P, Holzapfel GA (2011) Dissection properties and mechanical strength of tissue components in human carotid bifurcations. Ann Biomed Eng 39:1703–1719
Avdic T, Franzeán S, Zarrouk M, Acosta S, Nilsson P, Gottsäter A, Svensson AM, Gudbjörnsdottir S, Eliasson B (2018) Reduced long-term risk of aortic aneurysm and aortic dissection among individuals with type 2 diabetes mellitus: a nationwide observational study. J Am Heart Assoc 7:e007618
Jiménez-Trujillo I, González-Pascual M, Jiménez-Garća R, Hernández-Barrera V, Miguel-Yanes JM, Méndez-Bailón M, de Miguel-Diez J, Salinero-Fort MÁ, Perez-Farinos N, Carrasco-Garrido P, Lopez-De-Andrés A (2016) Type 2 diabetes mellitus and thoracic aortic aneurysm and dissection: an observational population-based study in Spain from 2001 to 2012. Medicine 95:e3618
Prakash SK, Pedroza C, Khalil YA, Milewicz DM (2012) Diabetes and reduced risk for thoracic aortic aneurysms and dissections: a nationwide case-control study. J Am Heart Assoc 1:e000323
He X, Liu X, Liu W, Wang B, Liu Y, Li Z, Wang T, Tan R, Gao B, Zeng H (2015) Association between diabetes and risk of aortic dissection: a case-control study in a Chinese population. PLoS One 10:e0142697
Theivacumar NS, Stephenson MA, Mistry H, Valenti D (2014) Diabetics are less likely to develop thoracic aortic dissection: a 10-year single-center analysis. Ann Vasc Surg 28:427–432
Takagi H, Umemoto T (2017) Negative association of diabetes with thoracic aortic dissection and aneurysm. Angiology 68:216–224
Liu H, Shi L, Zeng T, Ji Q, Shi Y, Huang Y, Zhang L, Xiao T, Ye J, Lin Y, Liu L (2019) Type 2 diabetes mellitus reduces clinical complications and mortality in Stanford type B aortic dissection after thoracic endovascular aortic repair: a 3-year follow-up study. Life Sci 230:104–110
Brownlee M, Cerami A, Vlassara H (1988) Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med 318:1315–1321
Sowers JR, Epstein M, Frohlich ED (2001) Diabetes, hypertension, and cardiovascular disease an update. Hypertension 37:1053–1059
Wolff SP, Jiang ZY, Hunt JV (1991) Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med 10:339–352
Vishwanath V, Frank KE, Elmets CA, Dauchot PJ, Monnier VM (1986) Glycation of skin collagen in type I diabetes mellitus. Correlation with long-term complications. Diabetes 35:916–921
Vlassara H, Brownlee M, Cerami A (1986) Nonenzymatic glycosylation: role in the pathogenesis of diabetic complications. Clin Chem 32:B37–B41
Cameron JD, Bulpitt CJ, Pinto ES, Rajkumar C (2003) The aging of elastic and muscular arteries: a comparison of diabetic and nondiabetic subjects. Diabetes Care 26:2133–2138
Konova E, Baydanoff S, Atanasova M, Velkova A (2004) Age-related changes in the glycation of human aortic elastin. Exp Gerontol 39:249–254
Wang Y, Li H, Zhang Y (2018b) Understanding the viscoelastic behavior of arterial elastin in glucose via relaxation time distribution spectrum. J Mech Behav Biomed Mater 77:634–641
Wang Y, Zeinali-Davarani S, Davis EC, Zhang Y (2015) Effect of glucose on the biomechanical function of arterial elastin. J Mech Behav Biomed Mater 49:244–254
Winlove CP, Parker KH, Avery NC, Bailey AJ (1996) Interactions of elastin and aorta with sugars in vitro and their effects on biochemical and physical properties. Diabetologia 39:1131–1139
Zou Y, Zhang Y (2012) The biomechanical function of arterial elastin in solutes. J Biomech Eng 134:071002
Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ (1996) The role of glycation cross-links in diabetic vascular stiffening. Diabetologia 39:946–951
De D, Pawar N, Gupta AN (2020) Glucose-induced structural changes and anomalous diffusion of elastin. Colloids Surf B: Biointerfaces 188:110776
Silverstein MC, Bilici K, Morgan SW, Wang Y, Zhang Y, Boutis GS (2015) 13C, 2H NMR studies of structural and dynamical modifications of glucose-exposed porcine aortic elastin. Biophys J 108:1758–1772
Barenblatt GI (1962) The mathematical theory of equilibrium cracks in brittle fracture. Adv Appl Mech 7:55–129
Dugdale DS (1960) Yielding of steel sheets containing slits. J Mech Phys Solids 8:100–104
Zou Y, Zhang Y (2009) An experimental and theoretical study on the anisotropy of elastin network. Ann Biomed Eng 37:1572–1583
Smart EJ, Ying YS, Donzell WC, Anderson RGW (1996) A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem 271:29427–29435
Kendall K (1975) Thin-film peeling-the elastic term. J Phys D Appl Phys 8:1449–1452
Parmigiani JP, Thouless MD (2006) The roles of toughness and cohesive strength on crack deflection at interfaces. J Mech Phys Solids 54:266–287
Chow MJ, Turcotte R, Lin CP, Zhang Y (2014) Arterial extracellular matrix: a mechanobiological study of the contributions and interactions of elastin and collagen. Biophys J 106:2684–2692
Yu X, Turcotte R, Seta F, Zhang Y (2018a) Micromechanics of elastic lamellae: unravelling the role of structural inhomogeneity in multi-scale arterial mechanics. J R Soc Interface 15:20180492
Yu X, Wang Y, Zhang Y (2018b) Transmural variation in elastin fiber orientation distribution in the arterial wall. J Mech Behav Biomed Mater 77:745–753
Schellekens JCJ, De Borst R (1993) On the numerical integration of interface elements. Int J Numer Methods Eng 36:43–66
Gasser TC, Ogden RW, Holzapfel GA (2006) Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J R Soc Interface 3:15–35
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61:1–48
Holzapfel GA, Gasser TC, Stadler M (2002) A structural model for the viscoelastic behavior of arterial walls: continuum formulation and finite element analysis. Eur J Mech A Solids 21:441–463
Chen JS, Han W, Wu CT, Duan W (1997) On the perturbed Lagrangian formulation for nearly incompressible and incompressible hyperelasticity. Comput Methods Appl Mech Eng 142:335–351
Tong J, Xin YF, Xu X, Yang F, Zhang Z (2020) Effect of diabetes mellitus on the dissection properties of the rabbit descending thoracic aortas. J Biomech 100:109592
Leng X, Zhou B, Deng X, Davis L, Lessner SM, Sutton MA, Shazly T (2018) Experimental and numerical studies of two arterial wall delamination modes. J Mech Behav Biomed Mater 77:321–330
Wang Y, Johnson JA, Spinale FG, Sutton MA, Lessner SM (2014) Quantitative measurement of dissection resistance in intimal and medial layers of human coronary arteries. Exp Mech 54:677–683
Wang Y, Ning J, Johnson JA, Sutton MA, Lessner SM (2011) Development of a quantitative mechanical test of atherosclerotic plaque stability. J Biomech 44:2439–2445
Aronson D (2003) Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21:3–12
Brownlee M, Vlassara H, Cerami A (1984) Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med 101:527–537
Cerami A, Vlassara H, Brownlee M (1985) Protein glycosylation and the pathogenesis of atherosclerosis. Metabolism 34:37–44
Black LD, Brewer KK, Morris SM, Schreiber BM, Toselli P, Nagent MA, Suki B, Stone PJ (2005) Effects of elastase on the mechanical and failure properties of engineered elastin-rich matrices. J Appl Physiol 98:1434–1441
Hemmer PC, Hansen A (1992) The distribution of simultaneous fiber failures in fiber bundles. J Appl Mech 59:909–914
Pradhan S, Hansen A, Chakrabarti BK (2010) Failure processes in elastic fiber bundles. Rev Mod Phys 82:499–555
Yu X, Suki B, Zhang Y (2020) Avalanches and power law behavior in aortic dissection propagation. Sci Adv 6:eaaz1173
Stephen EA, Venkatasubramaniam A, Good TA, Topoleski LDT (2014) The effect of glycation on arterial microstructure and mechanical response. J Biomed Mater Res Part A 102:2565–2572
Tang Y, Ballarini R, Buehler MJ, Eppell SJ (2010a) Deformation micromechanisms of collagen fibrils under uniaxial tension. J R Soc Interface 7:839–850
Lillie MA, Gosline JM (1996) Swelling and viscoelastic properties of osmotically stressed elastin. Biopolymers 39:641–652
Gosline JM (1978) Hydrophobic interaction and a model for the elasticity of elastin. Biopolymers 17:677–695
Li B, Alonso DOV, Bennion BJ, Daggett V (2001) Hydrophobic hydration is an important source of elasticity in elastin-based biopolymers. J Am Chem Soc 123:11991–11998
Wang Y, Hahn J, Zhang Y (2018a) Mechanical properties of arterial elastin with water loss. J Biomech Eng 140:041012
Eisenberg RL, Bank WO, Hedgock MW (1981) Renal failure after major angiography can be avoided with hydration. AJR Am J Roentgenol 136:859–861
Fick JM, Espino DM (2011) Articular cartilage surface rupture during compression: investigating the effects of tissue hydration in relation to matrix health. J Mech Behav Biomed Mater 4:1311–1317
Werbner B, Spack K, O’Connell GD (2019) Bovine annulus fibrosus hydration affects rate-dependent failure mechanics in tension. J Biomech 89:34–39
Wu KS, van Osdol WW, Dauskardt RH (2006) Mechanical properties of human stratum corneum: effects of temperature, hydration, and chemical treatment. Biomaterials 27:785–795
Gregory DE, Bae WC, Sah RL, Masuda K (2014) Disc degeneration reduces the delamination strength of the annulus fibrosus in the rabbit annular disc puncture model. Spine J 14:1265–1271
Roccabianca S, Ateshian GA, Humphrey JD (2014a) Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol 13:13–25
Roccabianca S, Bellini C, Humphrey JD (2014b) Computational modelling suggests good, bad and ugly roles of glycosaminoglycans in arterial wall mechanics and mechanobiology. J R Soc Interface 11:20140397
Murdock K, Martin C, Sun W (2018) Characterization of mechanical properties of pericardium tissue using planar biaxial tension and flexural deformation. J Mech Behav Biomed Mater 77:148–156
Willershausen-Zönnchen B, Lemmen C, Hamn G (1991) Influence of high glucose concentrations on glycosaminoglycan and collagen synthesis in cultured human gingival fibroblasts. J Clin Periodontol 18:190–195
Kemeny SF, Cicalese S, Figueroa DS, Clyne AM (2013) Glycated collagen and altered glucose increase endothelial cell adhesion strength. J Cell Physiol 228:1727–1736
Nienaber CA, Clough RE (2015) Management of acute aortic dissection. Lancet 385:800–811
Acknowledgements
The authors would like to thank Dr. Xin Brown for assistance with the testing facility as well as Dr. Yunjie Wang for providing helpful research suggestions on glucose treatment procedures. This study was supported by a grant from the National Institute of Health (2R01HL098028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, R., Yu, X., Gkousioudi, A. et al. Effect of Glycation on Interlamellar Bonding of Arterial Elastin. Exp Mech 61, 81–94 (2021). https://doi.org/10.1007/s11340-020-00644-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11340-020-00644-y