Abstract
In order to produce cheap activation carbon for dioxin adsorption in waste incinerator, waste plywood, pinewood, and coconut shell were used as raw materials to produce activated carbon via steam gasification on a lab-scaled rotary reactor. The influence of temperature, steam flow rate, and activation time on activated carbon yield and properties was investigated. Experimental results show that at 800 years, with 30-min activation time and steam/char ratio equal to 2, the yield of plywood activated carbon reached 60.5%, which was much higher than pinewood. The iodine adsorption value was 943 mg/g, which is close to pinewood. The pore structure analysis results show that the activated carbon derived from pinewood and plywood is similar. The pores with diameter of 2–5 nm were well developed, which was suitable for dioxin adsorption in waste incinerator. In the end, the computational fluid dynamics (CFD) and discrete element method (DEM) were used for modeling and optimization of the rotary furnace. Calculation results show that internal friction between the particles or the degree of anisotropy of the particles will cause the particles in the furnace to form a larger accumulation inclination, which benefits the mixing of the material in furnace. The results show that both the Eulerian-Eulerian two-fluid method and the DEM can simulate the movement of particles in the rotary furnace.

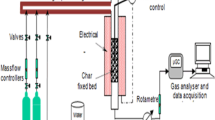
The activated carbon produced by steam gasification for dioxin adsorption.












Similar content being viewed by others
References
Tay T, Ucar S, Karagoz S (2009) Preparation and characterization of activated carbon from waste biomass. J Hazard Mater 165(1):481–485
Köseoğlu E, Akmil-Başar C (2015) Preparation, structural evaluation and adsorptive properties of activated carbon from agricultural waste biomass. Adv Powder Technol 26(3):811–818
Derlet RW, Albertson TE (1986) Activated charcoal--past, present and future. West J Med 145(4):493–496
Guo Y, Li Y, Zhu T, Wang J, Ye M (2016) Modeling of dioxin adsorption on activated carbon. Chem Eng J 283:1210–1215
Mukherjee A, Debnath B, Ghosh SK (2016) A review on technologies of removal of dioxins and furans from incinerator flue gas. Procedia Environ Sci 35:528–540
Kulkarni PS, Crespo JG, Afonso CAM (2008) Dioxins sources and current remediation technologies — a review. Environ Int 34(1):139–153
Long RQ, Yang RT (2001) Carbon nanotubes as superior sorbent for dioxin removal. J Am Chem Soc 123(9):2058–2059
Hajizadeh Y, Williams PT (2013) Activated carbon production from RDF and its use for dioxin removal from flue gas of waste incinerators. Int J Environ Health Eng 2(1):10
Czajczyńska D, Anguilano L, Ghazal H, Krzyżyńska R, Reynolds AJ, Spencer N, Jouhara H (2017) Potential of pyrolysis processes in the waste management sector. Therm Sci Eng Prog 3:171–197
Hirata T, Inoue M, Fukui Y (1992) Pyrolysis and combustion toxicity of wood treated with CCA. Wood Sci Technol 27(1):35–47
Fateh T, Rogaume T, Luche J, Richard F, Jabouille F (2014) Characterization of the thermal decomposition of two kinds of plywood with a cone calorimeter – FTIR apparatus. J Anal Appl Pyrolysis 107:87–100
Kim YH (2011) Process simulation of activated carbon production using a rotary kiln. Korean J Chem Eng 28:27–31
Miguel GS, Fowler GD, Dall’Orso M, Sollars CJ (2002) Porosity and surface characteristics of activated carbons produced from waste tyre rubber. J Chem Technol Biotechnol 77(1):1–8
Zhao Chuang WZ-X (2020) Application of a new type of internal heating activation furnace system in production. Chem Fertil Des 58(1):3
Zhe L (2003) Discussion on activated technology of SLEP furnace. TONG MEI KEJI 96:3
Yinw X (2014) Current status and development trend of coal-based activated carbon production equipment in China. Clean Coal Technol
Liu H, Yin H, Zhang M, Xie M, Xi X (2016) Numerical simulation of particle motion and heat transfer in a rotary kiln. Powder Technol 287:239–247
Sebastian Escotet-Espinoza M, Foster CJ, Ierapetritou M (2018) Discrete element modeling (DEM) for mixing of cohesive solids in rotating cylinders. Powder Technol 335:124–136
Hlosta J, Jezerská L, Rozbroj J, Žurovec D, Zegzulka J (2020) DEM Investigation of the influence of particulate properties and operating conditions on the mixing process in rotary drums: Part 2—Process Validation and Experimental Study. Processes 8(2):184
Zhao S, Zhang Y, Su Y (2019) Experimental investigation of rice straw oxidative pyrolysis process in a hot-rod reactor. J Anal Appl Pyrolysis 142:104646
Gonzalez MT, Molinasabio M, Rodriguezreinoso F (1994) Steam activation of olive stone chars, development of porosity. Carbon 32(8):1407–1413
Kang HS (2005) Theoretical study of binding of metal-doped graphene sheet and carbon nanotubes with dioxin. J Am Chem Soc 127(27):9839–9843
Wang R, Zhang D, Liu C (2017) DFT study of the adsorption of 2,3,7,8-tetrachlorodibenzo-p-dioxin on pristine and Ni-doped boron nitride nanotubes. Chemosphere 168:18–24
Wu FC, Tseng RL, Juang RS (2005) Comparisons of porous and adsorption properties of carbons activated by steam and KOH. J Colloid Interface Sci 283(1):49–56
Atkinson JD, Hung PC, Zhang Z, Chang M, Yan Z, Rood MJ (2014) Adsorption and destruction of PCDD/Fs using surface-functionalized activated carbons. Chemosphere 118C:136–142
Li W, Yang K, Peng J, Zhang L, Guo S, Xia H (2008) Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars. Ind Crop Prod 28(2):190–198
Bell JG, Zhao X, Uygur Y, Thomas KM (2011) Adsorption of chloroaromatic models for dioxins on porous carbons: the influence of adsorbate structure and surface functional groups on surface interactions and adsorption kinetics. J Phys Chem C 115(6):2776–2789
Liu Y, Su F-Y, Wen Z, Li Z, Yong H-Q, Feng X-H (2014) CFD Modeling of flow, temperature, and concentration fields in a pilot-scale rotary hearth furnace. Metall Mater Trans B Process Metall Mater Process Sci 45(1):251–261
Li D, Liu G, Lu H, Zhang Q, Wang Q, Yu H (2016) Numerical simulation of different flow regimes in a horizontal rotating ellipsoidal drum. Powder Technol 291:86–96
Komossa H, Wirtz S, Scherer V, Herz F, Specht E (2014) Transversal bed motion in rotating drums using spherical particles: comparison of experiments with DEM simulations. Powder Technol 264:96–104
Hlungwani O, Rikhotso J, Dong H, Moys MH (2003) Further validation of DEM modeling of milling: effects of liner profile and mill speed. Miner Eng 16(10):993–998
Henein H, Brimacombe JK (1983) The modeling of transverse solids motion in rotary kilns. Metall Urgical Trans B 14:14
Mellman J (2001) The transverse motion of solids in rotating cylinders-forms of motion and transition behavior. Powder Technol 118:20
Funding
This work was supported by Science Foundation of Nanjing Institute of Technology (YKJ201813)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhao, S., Chen, L. Utilization of biomass waste for activated carbon production by steam gasification in a rotary reactor: experimental and theoretical approach. Biomass Conv. Bioref. 12, 3943–3953 (2022). https://doi.org/10.1007/s13399-020-00921-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00921-9