So Uncommon and so Singular, but Underexplored: An Updated Overview on Ethnobotanical Uses, Biological Properties and Phytoconstituents of Sardinian Endemic Plants
Abstract
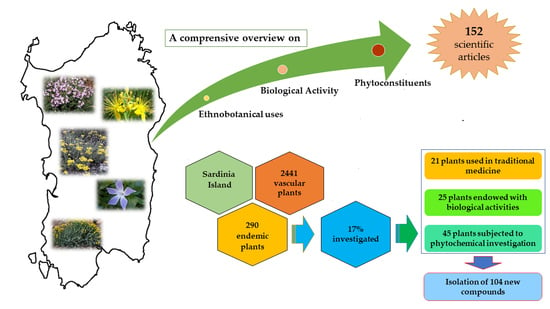
:1. Introduction
2. Methodology
3. Results and Discussion
3.1. Ethnobotanical Uses
| Taxon | Ethnobotanical Uses | Part(s) of the Plant Used | Preparation | Territories | References |
|---|---|---|---|---|---|
| Aristolochia thyrrena | Emmenagogue, vulnerary | WP | Infusion | Barbagia Seui, Seulo, Sadali | [30,32,33] |
| Arum pictum subsp. pictum | Vulnerary, burns and scalds | L, T | Cataplasm | Villagrande Strisaili, Barbagia Seui, Urzulei, Barbagia Seulo | [30,32,33,34,35] |
| Diuretic, nervous sedative | L | Decoction | Urzulei | [30,34] | |
| Haemostatic | L | Direct application | Tempio Pausania | [30,36] | |
| Anti-rheumatic | F | Cataplasm | Urzulei, Barbagia Seui, Barbagia Seulo | [30,32,34] | |
| Skin lightening | T | Latex | Villamassargia | [30] | |
| Cymbalaria muelleri | Burns, chilblains, skin inflammation, hemorrhoids | AP | Cataplasm | Laconi | [29,30] |
| Euphorbia pithyusa subsp. cupanii | Removal of warts | LX | Direct application | Barbagia Seui, Sadali, Seulo | [30,32,33] |
| Antiasthmatic | FP | Decoction | Barbagia Seui, Sadali, Seulo | [30,32] | |
| Analgesic | LX | Direct application | Bolotana | [30] | |
| Glechoma sardoa | Respiratory diseases, chronic catarrh, bronchitis, antiasthmatic, wound healing | F and S | Infusion with milk and honey | Laconi | [29,30] |
| Vulnerary, resolvent in scalds, antinevralgic, antirheumatic | F and S | Infusion, infusion with milk and honey (direct application) | Laconi | [29,30] | |
| Helichrysum italicum subsp. tyrrhenicum | Anti-allergic | WP | Infusion | Urzulei | [34] |
| Skin diseases (alopecia), diaphoretic, burns, vulnerary | WP, FH, S | Decoction, infusion, cataplasm | Fluminimaggiore, Gesturi, Tempio Pausania | [27,36,37] | |
| Bronchitis, laryngitis, tracheitis, cough sedative, expectorant | L, FH | Infusion | Barbagia Seui, Laconi, Escolca, Tempio Pausania | [29,32,36,38] | |
| Antineuralgic, antirheumatic | FH | Infusion | Barbagia Seui, Laconi, Tempio Pausania | [29,32,36] | |
| Hypericum hircinum subsp. hircinum | Burns and wound healing | F | Macerate in olive oil | Laconi | [29,30] |
| Antirheumatic, sciatica, dislocation | F | Macerate in olive oil and wine | Laconi | [29,30] | |
| Balsamic, antiasthmatic | WP | Infusion | Laconi | [29,30] | |
| Pancratium illyricum | Depurative, diuretic, emetic, corn removal, antiseptic | B | Powder, cataplasm | Tonara, Iglesiente | [30] |
| Polygala sardoa | Fluidifyng | R | Decoction | Laconi | [29,30] |
| Ptilostemon casabonae | Antispasmodic | AP | Ingestion | Urzulei | [30] |
| Salvia desoleana | Antipyretic, anti-inflammatory | S and L | Decoction | Loceri | [30] |
| Vulnerary | L | Cataplasm | Loceri | [30] | |
| External anti-inflammatory | L | Heated in oil | Villanovaforru | [30] | |
| Santolina corsica | Anthelmintic, tonic, emmenagogue, insect repellent | AP | Not available information | Lodè, Lula | [30] |
| Santolina insularis | Insect repellent (pediculus) | WP, S, L | Fumigation | Arzana, Villagrande Strisaili | [35,39] |
| Anthelmintic | FH, L | Infusion | Marganai, Laconi | [29,40] | |
| Febrifuge, sedative, antitussive | L | Decoction | Villagrande Strisaili | [35] | |
| Scrophularia trifoliata | Skin diseases, vulnerary, anti-edema, antirheumatic | L, FH, RH | Infusion, cataplasm of fresh leaves with olive oil, cream | Ussassai, Urzulei, Villagrande Strisaili, Escolca | [28,33,34,35,38] |
| Diuretic | L | Decoction | Escolca | [38] | |
| Emollient | R | Direct application | Barbagia Seui, Sadali, Seulo | [30,32,33] | |
| Anthelmintic | R | Powder with honey | Barbagia Seui, Sadali, Seulo | [30,32] | |
| Purgative, emetic, Basedow’s disease and related heart disorders | L | Infusion | Gesturi | [27] | |
| Antirheumatic, vurnerary | L | Cataplasm | Urzulei | [30] | |
| Antipyretic, anti-inflammatory, tonsillitis, sore throat | L | Cataplasm | Aggius | [30] | |
| Stachys glutinosa | Hepatoprotective, cholagogue, diuretic | L | Decoction | Gesturi | [27] |
| Common cold | L | Decoction | Villagrande Strisaili | [35] | |
| Antiseptic, antispasmodic | WP | Infusion | Arzana | [30,33,39] | |
| Sedative | WP | Infusion | Bolotana | [30] | |
| Staphisagria requienii subsp. picta | Antiparasitic (lice, nits, mites), vulnerary | L, SE | Ointment, powder | Barbagia Seui, Bolotana | [30,32] |
| Tanacetum audibertii | Digestive, anthelmintic, anti-arthritic, emmenagogue | AP | Decoction | Not available information | [30] |
| Thymus herba-barona subsp. herba barona | Anthelmintic, digestive, to treat gastralgia | WP, S, L, R | Decoction, infusion | Arzana, Urzulei, Laconi | [29,34,39] |
| Antirheumatic | WP | Cataplasm | Urzulei | [34] | |
| Cough sedative, whooping cough, expectorant | L, F | Infusion with milk, decoction | Arzana, Ussassai, Laconi | [28,29,39] | |
| Antipyretic | WP | Decoction with malva and lemon, soaked in “grappa” | Arzana, Urzulei | [34,39] | |
| Sore throat, common cold, cough, bronchitis, asthma, tonic, antianaemic, intestinal antispasmodic, diuretic | L, S | Decoction, inhalation of infusion with malva, rosemary and sage | Arzana, Villagrande Strisaili, Laconi, Urzulei, Perdasdefogu | [29,34,35,39,41] | |
| Cold | R and F | Inhalation of decoction | Arzana | [39] | |
| Antiseptic, tonic, disinfectant, mouthwash | L, L and R | Infusion, decoction | Perdasdefogu, Arzana | [33,39,41] | |
| Urticaria, foot perspiration | WP | Powder | Perdasdefogu | [33,41] | |
| Lenitive | L | Cataplasm | Ussassai | [28] | |
| Toothache | R | Chewing | Arzana | [39] | |
| Urtica atrovirens | Baldness, rashes, vulnerary, hemostatic, antirheumatic, emmenagogue, gastralgia | L, WP | Infusion | Lotzorai, Marganai, Domusnovas | [30,38,40] |
| Diuretic | WP, L | Infusion, syrup | Barbagia Seui, Marganai, Seulo, Sadali | [30,32,40] | |
| Verbascum conocarpum subsp. conocarpum | Anti-inflammatory, anti-catarrhal | L | Decoction, fumigation | La Maddalena Archipelago | [30] |
| Vinca difformis subsp. sardoa | Anti-rheumatic | L | Application of heated leaves | Ussassai | [42] |
| Sedates nausea | L | Infusion | Escolca | [38] | |
| Anti-emetic, eupeptic, anti-inflammatory, anti-hemorragic, galactofuge, astringent, hypotensive, hypoglycaemic | L | Decoction, infusion | Laconi, Perdasdefogu, Escolca, Monteleone | [29,38,41,42] | |
| Antitubercular | L | Infusion, maceration | Monteleone | [42] | |
| Bronchitis | L | Poultice | Lodine | [43] |
3.2. Pharmacological Activities
3.2.1. Antimicrobial Activity
3.2.2. Antiviral Activity
3.2.3. Anticancer Activity
3.2.4. Antioxidant Activity
3.2.5. Anti-Inflammatory Activity
3.2.6. Anti-Aging Activity
3.2.7. Estrogenic/Antiestrogenic Activity
3.2.8. Antidiabetic Activity
3.2.9. Other Activities
3.3. Phytoconstituents
| Taxon | Type of Extract | Plant Organ | Main Constituents | Voucher Specimen | References |
|---|---|---|---|---|---|
| Artemisia caerulescens subsp. densiflora | Essential oil | Flowers | Camphor, isoborneol, terpinen-4-ol, camphene | N.A. | [191] |
| Essential oil | Leaves | α-thujone, terpinen-4-ol, camphor | N.A. | [191] | |
| Essential oil | Aerial parts | Terpinen-4-ol, p-cymene, ʏ-terpinene, 1,8-cineole, α-terpineol | Herbarium SASSA 736 | [46] | |
| Essential oil | Aerial parts | Davana ethers, cis-Sabinene hydrate, terpinen-4-ol, (E)-nerolidol, β-Oplopenone | Herbarium CAG 736 | [98] | |
| Ethanol extract | Aerial parts | α/β dihydroartemisinin, artemisinin, artemisic acid, absinthin, phytol, stigmasterol, 3-O-caffeoylquinic acid, caffeic acid, 3-O-caffeoyl-5-O-feruloylquinic acid, isofraxidin, apigenin, quercetin 3-methyl ether, eupalitin, luteolin-7-O-methyl ether, axillarin and cirsimaritin | Herbarium CAG 736 | [201] | |
| Astragalus verrucosus | Ethyl acetate and n-buthanol extracts | Aerial parts | Saponins: astraverrucins I **, II **, III **, IV **, V **, VI **; D-pinitol | URB-96/357 | [202,203,204] |
| Ethyl acetate and n-buthanol extracts | Aerial parts | Saponins: astraverrucin VII **, cycloaraloside D (peregrinoside II) and cycloaraloside C (astrailienin A) | URB-96/357 | [204] | |
| Chloroform, ethyl acetate and n-buthanol extracts | Aerial parts | Flavonoids: Daidzein, genistein, apigenin, apigenin 7-glucoside, nicotiflorin, rutin, apigenin 7-O-β-D-(6-Op-coumaroyl)glucoside, kaempferol 3-O-robinobioside, quercetin 3-O-robinobioside, daidzin, ononin, calycosin, psudobaptigenin, genistin, pratensein | URB-96/357 | [204] | |
| Chloroform, ethyl acetate and n-buthanol extracts | Aerial parts | Pterocarpan derivative: maackiain | URB-96/357 | [204] | |
| Bituminaria morisiana | Acetone extract | Aerial parts | Erybraedin C, bitucarpin A **; 8-prenyldaidzein, plicatin B | Herbarium CAG 391/B | [205] |
| Petroleum ether, ethyl acetate and methanol extracts | Aerial parts | Erybraedin C, plicatin B, and bitucarpin A | Herbarium CAG 391/B | [130] | |
| Petroleum ether and ethyl acetate extracts | Leaves | Erybraedin C, bitucarpin A, 3,9-dihydroxy-4-(3,3-dimethyallyl)[6aR,11aR]-pterocarpan **, 3-hydroxy-4-(3,3-dimethylallyl)-4″,5″-dehydropyrano[8,9:2″,3″][6aR,11aR]-pterocarpan **, 4′,5″-dihydroxy-6″-methoxy-4″,4″-dimethyl-4″,5″-dihydro-6″H-pyrano[7,8:2″,3″]-isoflavone **, daidzein, 8-prenyldaidzein, bidwillon C, coumestrol, pseudoisopsoralen | Herbarium of Dipartimento Farmaco Chimico Tecnologico, University of Cagliari (No. 0201) | [102] | |
| Ethyl acetate extract | Seeds | 3-hydroxy-4-(3′-methyl-2′-butenyl)-furo[2′,3′:8,9][6aR,11aR]pterocarpan (morisianine) **, erybraedin C, psoralen, angelicin | Herbarium of Dipartimento Farmaco Chimico Tecnologico, University of Cagliari (No. 0201) | [103] | |
| Borago morisiana | Fatty acid methyl esters (FAME) | Seeds | γ-linoleic and stearidonic acids | HUAL 25639 | [206] |
| HUAL 25965 | |||||
| Borago pygmaea | Fatty acid methyl esters (FAME) | Seeds | γ-linoleic and stearidonic acids | HUAL 25608 | [206] |
| Centaurea horrida | Methanol extract | Aerial parts | Flavonoid glycoside: horridin ** | URB-3214/97 | [207] |
| N-hexane, chloroform, chloroform-methanol and methanol extracts | Aerial parts | Lupeol, betulin, apigenin, 5-caffeoylquinic acid, β-sitosterol 3-O-β-D-glucopyranoside, rutin, 3-caffeoylquinic acid, apigenin 3-O-β-D-glucopyranouronide, apigenin 8-C-α-1-arabinopyranoside, apigenin 6-C-α-L-arabinopyranoside, protocatechuic acid, scutellarein 7-O-β-D-glucopyranoside, quercetin 3-O-α-L-rhamnopyranoside, apigenin 7-O-β-D-glucopyranoside, kaempferol 3-O-β-D-glucopyranoside, kaempferol 3-O-α-L-rhamnopyranoside, horridin, cis- and trans-3,5-dicaffeoyl quinic acids, vitexin, isovitexin, orientin, shaftoside, apigenin 6,8-di-C-β-D-glucopyranoside and 4-caffeoylquinic acid | Herbarium Horti Botanici Pisani 03/7219 | [208] | |
| Cymbalaria muelleri | Ethanol extract | Aerial parts | Iridoid glycosides: antirrhinoside, antirrhide, macfadienoside, 7-β -hydroxy 8-harpagide | N.A. | [209] |
| Euphorbia hyberna subsp. insularis | Acetone extract | Aerial parts | Jatrophane diterpenoids | N.A. | [210] |
| Euphorbia pithyusa subsp. cupanii | Acetone extract | Aerial parts | Lathyrol-3-phenylacetate-5,15-diacetate **, premyrsinol-3-propanoate-5-isobutyrate-7,13,17-triacetate **, premyrsinol-3-propanoate-5-isobutyrate-7,13-diacetate-17-nicotinate **, premyrsinol-3-propanoate-5(R-methyl)butyrate-7,13-diacetate-17-isobutyrate **, premyrsinol-3-propanoate-5,17-diisobutyrate-7,13-diacetate **, premyrsinol-3,17-dipropanoate-5-isobutyrate-7,13-diacetate **, premyrsinol-3-propanoate-5-benzoate-7,13,17-triacetate **, premyrsinol-3-propanoate-5-isobutyrate-7,13,17-triaacetate **, 4,12,20-trideoxyphorbol-13-(2,3-dimethyl)butyrate **, 4,12-dideoxyphorbol-13-(2,3-dimethyl)butyrate **, 4,12-dideoxyphorbol-13-(2,3-dimethyl)butyrate-20-acetate ** | Herbarium CAG 1212 | [197] |
| Fatty acid methyl esters (FAME) | Seeds | palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid | Herbarium CAG 1212 | [131] | |
| Unsaponifiable fraction | Seeds | hydrocarbons, fatty alcohols, campesterol, β-sitosterol, Δ5-avenasterol, cycloartanol, 24-methylen-cycloartenol | Herbarium CAG 1212 | [131] | |
| Tocopherols | Seeds | α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol | Herbarium CAG 1212 | [131] | |
| Euphorbia semiperfoliata | Ethyl acetate extract * | Whole plant | Eleven Jatrophane esters **, three 4-deoxyphorbol esters ** | [81,82,199] | |
| Acetone extract | Aerial parts | Scopoletin, helioscopinolides A and B, an abietanolide **, 13 jatrophane polyesters **, two 4-deoxyphorbol diesters **, 2 epimeric diterpenes ** | Herbarium CAG 1217 | [198] | |
| Acetone extract | Aerial parts | Jatrophane polyesters | N.A. | [104] | |
| Fatty acid methyl esters (FAME) | Seeds | myristic acid, palmitic acid, stearic acid, oleic acid, linoleic acid, linolenic acid, arachidic acid, behenic acid | Herbarium CAG 1217 | [131] | |
| Unsaponifiable fraction | Seeds | hydrocarbons, fatty alcohols, cholesterol, campesterol, β-Sitosterol, lanosterol isomer, lanosterol, β-amyrin, cycloartanol, 24-Methylen-cycloartenol | Herbarium CAG 1217 | [131] | |
| Tocopherols | Seeds | α-tocopherol, β-tocopherol, γ-tocopherol, δ-tocopherol | Herbarium CAG 1217 | [131] | |
| Euphrasia nana | Ethanol extract of aerial parts | Aerial parts | Iridoid glucosides: aucubin, catalpol, mussaenosidic acid and melampyroside | N.A. | [211] |
| Ferula arrigonii | N.A. | N.A. | 7,11-dehydrogrilactone * (jalcaguaianolide derivative) | N.A. | [212] |
| Acetone extract | Roots | Coumarin derivatives: colladonin, colladin, badrakemone, umbelliprenin, kataravicinol, isosamarkandin angelate; daucane esters: ferutidin, lapiferin, 2α-Hydroxiferutidin **, 2-oxoferutidin **, latifolone | N.A. | [106] | |
| Acetone extract | Fruits | Lapiferin, ferutidin, Jaeskeanadiol veratrate, webbiol angelate, 10α-hydroxyferutidin, 10-deangeloylpallinin **, laserin | N.A. | [106] | |
| Galium corsicum | Ethanol extract | Aerial parts | Iridoids: asperuloside, monotropein, asperulosidic acid, scandoside, loganic acid; coumarin | Herbarium CAG 652 | [213] |
| Galium glaucophyllum | Ethanol extract | Aerial parts | Iridoids: asperuloside, monotropein, asperulosidic acid, deacetyl-asperuloside, geniposidic acid, loganin and loganic acid | Herbarium CAG 654 | [213] |
| Galium schmidii | Ethanol extract | Aerial parts | Iridoids: asperuloside, monotropein, geniposidic acid, loganin and 10-hydroxy-loganin | Herbarium CAG 654/a | [213] |
| Genista arbusensis | Essential oil | Flowers | Heptanal, 1-octen-3-ol, (E,Z)-2,6-nonadienal, (E)-2-(2-pentenyl)-furan, 2-penthylfuran, (E)-2-hexenal | GA120503NE | [196] |
| Genista bocchierii | Essential oil | Flowers | Caryophyllene oxide, 1-octen-3-ol, heptanal, β-cariophyllene, n-pentadecane, (E,Z)-2,6-nonadienal | GB240504PU | [196] |
| Genista cadasonensis | Essential oil | Flowers | 1-octen-3-ol, (E)-2-(2-pentenyl)-furan, linalool, 2-penthylfuran, (E,Z)-2,6-nonadienal | GD290403CS | [196] |
| Ethanol extract | Aerial parts | Flavonoids: luteolin, genistein and 6-hydroxy-genistein | Herbarium CAG 290/A | [132] | |
| Ethanol extract | Fruits | Pinitol, 3-methoxy-chiro-inositol | Herbarium CAG 290/A | [132] | |
| Genista corsica | Essential oil | Flowers | (E,Z)-2,6-nonadienal, 1-octen-3-ol, E-β-farnesane, (E)-2-hexenal, (E)-2-nonenal, mesitylene | GC230402CA | [196] |
| Ethyl acetate extract | Leaves | Daidzein and luteolin | N.A. | [214] | |
| N.A. | N.A. | Quinolizidine alkaloids: anagyrine, cytisine, N-methylcytisine, lupanine, retamine and sparteine | N.A. | [215] | |
| N-hexane, choloroform and methanol extracts | Aerial parts | Dihydroisoderrondiol **, daidzein, luteolin, luteolin 4′-O-β-glucoside, luteolin 7-O-β-glucoside, isoprunetin, isoderrone, ficuisoflavone, taxifolin, 5-methoxytaxifolin, sucrose, D-pinitol | URB-1742/96 | [216] | |
| Genista ephedroides | N-hexane, choloroform and methanol extracts | Aerial parts | Hydroxyalpinumisoflavone **, ephedroidin **, genisteon **, genistein, isoprunetin, wighteone, laburnetin, alpinumisoflavone, genistin, genistein 8-C-glucoside, apigenin, isokaempferide, licoflavone C, pinitol | URB-167/94 | [217] |
| N.A. | N.A. | Alkaloids: sparteine, lupanine, anagyrine, cytisine, N-methylcytisine, retamine | N.A. | [215] | |
| N-hexane, choloroform and methanol extracts | Aerial parts | Alkaloids: lupanine, anagyrine, 17-oxoretamine, 12-α-hydroxylupanine, retamine | URB-167/94 | [218] | |
| N-hexane, choloroform and methanol extracts | Aerial parts | Licoflavone C | N.A. | [125,164,165] | |
| Genista morisii | Essential oil | Flowers | n-pentacosane, (E)-2-(2-pentenyl)-furan, (E)-2-hexenal, 2-penthylfuran, (E,Z)-2,6-nonadienal, E-β-farnesane | GM080403SA | [196] |
| Hydrolyzed extracts | Leaves | Flavonoids: daidzein, genistein, isoprunetin, luteolin | N.A. | [214] | |
| N-hexane, choloroform and methanol extracts | Aerial parts | Flavonoids: Genistein, daidzein, isoprunetin, eriodictyol, genistein-7-O-β-D-glucopyranoside, isoprunetin 7-O-β-D-glucopyranoside, vitexin, orientin, luteolin, luteolin 7-O-β-D-glucopyranoside, isoprunetin 7,4′ -di-O-β-D-glucopyranoside, genistein 7,4′-di-O-β-D-glucopyranoside | Herbarium SASSA 287 | [219] | |
| N-hexane, choloroform and methanol extracts | Aerial parts | Flavonoids: daidzein, genistein, genistein 7-O-β-D-glucopyranoside, isoprunetin, isoprunetin 7-O-β-D-glucopyranoside, isoprunetin 4′,7-di-O-β-D-glucopyranoside, luteolin, luteolin 7-O-β-D-glucopyranoside, luteolin 4′-O-β-D-glucopyranoside | N.A. | [164,165] | |
| Genista pichisermolliana | Essential oil | Flowers | β-myrcene, nerol, (E)-2-(2-pentenyl)-furan, γ-curcumene, linalool, nonanal, neryl acetate | GP130603LA | [196] |
| Petroleum ether, chloroform and methanol extracts | Aerial parts | Alpinumisoflavone 4′-O-glucopyranoside **, daidzein, genistein, genistein 7-O-β-glucopyranoside, biochanin A 7-O-β-glucopyranoside, genistein 4′,7-di-O-β-glucopyranoside, genistein 8-C-β-glucopyranoside, orobol 8-C-β-glucopyranoside, 3′-O-methylorobol 8-C-β-glucopyranoside, rutin, quercetin 3-O-robinobioside, isorhamnetin 3-O-β-glucopyranoside, isorhamnetin 3-O-β-galattopyranoside, isorhamnetin 3-O-robinobioside, apigenin, luteolin 7-O-β-glucopyranoside, eriodictiol, aromadendrin 7-O-β-glucopyranoside, maackiain, 4-methoxymaackiain, p-coumaric methylester and D-pinitol | Herbarium CAG 290/b | [220] | |
| Genista sulcitana | Essential oil | Flowers | 1-octen-3-ol, (E)-2-hexenal, β-myrcene, (E,Z)-2,6-nonadienal, (E)-2-(2-pentenyl)-furan, 3-octanol | GS090503MV | [196] |
| Methanol extract | Aerial parts | luteolin, 7-O-glucoside, genistein 7-O-glucoside, genistein 8-C-glucoside, p-coumaric acid | N.A. | [221] | |
| Glechoma sardoa | Essential oil | Aerial parts | β-elemene, δ-elemene, γ-elemene, isogermafurene | URB-GS 165 | [51] |
| Essential oil | Aerial parts | germacrene D, β-elemene, isogermafurene, δ-elemene, β-phellandrene, elemol, γ-elemene, δ-elemene | N.A. | [222] | |
| Helichrysum italicum subsp. tyrrhenicum | Essential oil | Aerial parts | Neryl acetate, nerol, neryl propionate, linalool, rosifoliol, γ -curcumene, γ-cadinene, δ-cadinene | Herbarium SASSA 729 [53,192,193] | [52,53,97,192,193,223,224,225,226] |
| Herbarium CAG 729 [97] | |||||
| Methanol extract | Aerial parts | α-terpinolene, trans-cariophyllene and neryl acetate) | N.A. | [54] | |
| Acetone extract | Aerial parts | Arzanol (phloroglucinol α-pyrone), oleyl ω-hydroxylinalol, helipyrone, tremetones, mycropyrone | Herbarium CAG 729 | [83] | |
| Acetone extract | Aerial parts | Arzanol, methylarzanol, helipyrone, micropyrone, rosifoliol, 10-hydroxytremetone, acetoxytremetone, acetoxyhydroxytremetone | N.A. | [101,134,135] | |
| Acetone extract | Aerial parts | Arzanol | N.A. | [133] | |
| Acetone extract | Aerial parts | Arzanol, ursolic acid, neryl acetate, ω-oleoyloxylinalol, two O-geranylated isomeric coumarates, helipyrone, oleoylbitalin A **, nonanoylbitalin A **, propanoylbitalin A **, isocaproylbitalin A **, six angeloylated lipids (santinols) **, micropyrone, heliarzanol ** | Herbarium CAG 729/10 | [57] | |
| Acetone extract | Aerial parts | Micropyrone, arzanol, helipyrone, two acylic derivatives of bitalin A, gnaphaliol, caffeic acid, ursolic acid, 7-O-β-(D-glucopyranosyl)-5-methoxy-1(3H)-isobenzofuranone, gnaphaliol-9-O-β-D-glucopyranoside, gnaphaliol-3-O-β-D-glucopyranoside, 6-O-β-(D-glucopyranosyl)-4-methoxy-1(3H)-benzofuranone ** | Herbarium CAG 729 | [200] | |
| Helichrysum saxatile subsp. saxatile | Essential oil | Flowers | α-pinene, limonene, 1,8-cineole, γ-curcumene. β-caryophyllene | HMGBH.e/9006.2015.009 | [227] |
| Hypericum hircinum subsp. hircinum | Methanol extract | Leaves | Quercetin, eriodictyol, 1,6-dihydroxy-5,7-dimethoxyxanthone, (4R)-4-hydroxy-5,5-dimethyldihydrofuran-2-one, quercetin-3′-O-β-D-glucopyranoside and eriodictyol-7-O-β-D-glucopyranoside | Herbarium CAG 0201 | [182] |
| Hydroalcoholic and ethanol extracts | Aerial parts | Betulinic acid, shikimic acid, chlorogenic acid, quercetin, 5,7,3′,5′-tetrahydroxyflavanone, 5,7,3′,5′-tetrahydroxyflavanone-7-O-glucoside | Herbarium CAG 232 | [84,136] | |
| Hydroalcoholic and ethanol extracts | Aerial parts | Shikimic acid, chlorogenic acid, quercetin, quercetin-7-O-glucoside, hypericin | Herbarium CAG 232 | [137] | |
| Hypericum scruglii | Hydro alcoholic and ethanol extracts | Aerial parts | Shikimic acid, chlorogenic acid, quercitrin, 3-geranyl-1-(2′-methylbutanoyl)-phloroglucinol, 3-geranyl-1-(2′-methylpropanoyl)-phloroglucinol, hyperoside, hypericin | Herbarium CAG 239/c | [137] |
| Hydroalcoholic extract | Leaves | 3-geranyl-1-(2′-methylbutanoyl)phloroglucinol, 3-geranyl-1-(2′-methylpropanoyl)phloroglucinol, 3-(13-hydroxygeranyl)-1-(2′-methylbutanoyl)phloroglucinol **, 1,3,5-benzentriol 2-[(2S,3R)-3-(3,4-dihydroxylphenyl)-2,3-dihydroxylpropyl], 3,4-dihydroxybenzoic acid and quercitrin | Herbarium CAG 239/c | [86] | |
| Limonium contortirameum | Aqueous extract | Aerial parts | Quinic acid, gallic acid, (+)-catechin, quercetin 3-O-[6-(3 hydroxy-3-methyl-glutaroyl)B-D-galactoside, phlorizin, epigallocatechin, phloretin, epigallocatechin-3-gallate, 6″-galloylmyricetin-3-O-β-D-galactopyranoside, 6″-galloylmyricetin-3-O-β-D-glucoside, quercetin 3,4′-diglucoside, myricetin 3-O-β-D-glucopyranoside, myricetin 3-O-rhamnoside, myricetin 3-O-arabinopyranoside, quercetin 3-glucoside, myricetin 3-O-xylopyranoside, myricetin | Herbarium SASSA 909 | [174] |
| Limonium morisianum | Methanol extract | Aerial parts | Myricetin, myricetin3-O-rutinoside, myricetin-3-O-(6″-O-galloyl)-β-D-galactopyranoside, (-)-epigallocatechin 3-O-gallate, tryptamine, ferulic and phloretic acids | Herbarium CAG 909/G | [88] |
| Methanol extract | Aerial parts | Myricetin, (-)-epigallocatechin 3-O-gallate | Herbarium CAG 909/G | [90] | |
| Linaria flava subsp. sardoa | N.A. | Aerial parts | Iridoid glucosides: 6′-O-acetylantirrhinoside **, antirrhinoside, 5-deoxyantirrhinoside, 5-glucosylantirrhinoside, antirrhide, linarioside, linaride, arcusangeloside | N.A. | [228] |
| Mentha requienii subsp. requienii | Essential oil | Aerial parts | Oxygenated monoterpenes: pulegone, isomenthone, isopulegone, limonene, linalool | Herbarium SASSA 1074 [47] | [47,229] |
| Pancratium illyricum | Methanol extract | Bulbs | Alkaloids: ungeremine, (−)-lycorine, (+)-vittatine | N.A. | [61] |
| Dichloromethane extract | Bulbs | 7,3′-dihydroxy-4′-methoxy-8-methyl flavan, 7,3′-dihydroxy-4′-methoxy flavan, p-hydroxyphenethyl trans-ferulate and sucrose | N.A. | [61] | |
| Methanol extract | Bulbs | Alkaloids: lycorine, 2-hydroxyhomolycorine, vittatine, galanthamine, sanguinine, habranthine | Herbarium CAG 1365 | [185] | |
| Methanol extract | Leaves | Alkaloids: leucotamine, O-methylleucotamine, 11α-hydroxy-O-methylleucotamine ** | Herbarium CAG 1365 | [185] | |
| Methanol extract | Bulbs and Leaves | Alkaloids: galanthamine, sanguinine, vittatine, habranthine, lycorine, leucotamine, O-methylleucotamine, 2-hydroxyhomolycorine | Herbarium CAG 1365 | [62] | |
| Plagius flosculosus | Ethyl acetate extract | Aerial parts | 3 polyacetylene spiroketals | N.A. | [149] |
| Dichloromethane extract | Leaves | Flosculin A **, flosculin B **, flosculin C ** and other five diacetylenic spiroketal enol ethers | Herbarium CAG 0311 | [109] | |
| Essential oil | Leaves | β-phellandrene, myrcene, iso-3-thujanol, (Z)-β-farnesene, β-pinene | Herbarium CAG 743 | [64] | |
| Super critical Fluid Extract | Leaves | (Z)-β-farnesene, β-phellandrene, myrcene | Herbarium CAG 743 | [64] | |
| Ptilostemon casabonae | Hydroalcoholic extracts | Aerial parts | Quercetin, luteolin, kaempferol, apigenin and diosmetin O-glycosides, and caffeoylquinic acid derivatives | N.A. | [230] |
| Salvia desoleana | Essential oil | Leaves | Linalyl acetate, α-terpinil acetate, 1,8-cineole, α-terpineol, linalool, α-phellandrene, limonene, β-pinene, geranyl acetate, trans-linalool oxide, sabinene, geraniol, α-pinene | N.A. | [66,184] |
| Essential oil | Leaves | Linalyl acetate, α-terpinil acetate, 1,8-cineole, linalool, germacrene D, α-terpineol, β-pinene, sabinene, sclareol, β-myrcene, limonene, α-pinene | N.A. | [65] | |
| Essential oil | Leaves | Germacrene D, α-terpinil acetate, sclareol, 1,8-cineole, linalool | N.A. | [138] | |
| Super critical fluid extract | Leaves | Sclareol, dotriacontano, vitamin E, nonacosane, terpenyl acetate, germacrene D, 1,8-cineole | N.A. | [138] | |
| Essential oil | Leaves | (Z)-β-Ocimene, α-Terpinyl acetate, 1,8-cineole, myrcene | N.A. | [231] | |
| Essential oil | Leaves | Samples from Gesturi: germacrene D, 1,8-cineole, α-terpinolene, α-terpinil acetate, bicyclogermacrene, α-pinene, sabinene, β-pinene, α-phellandrene. Samples from Luogosanto, Nuragus, Saccheddu, Villagrande: linalyl acetate, α-terpinil acetate, 1,8-cineole, germacrene D, linalool, β-myrcene, β-pinene | N.A. | [232] | |
| Essential oil | Aerial parts | Linalyl acetate, germacrene D, α-terpinil acetate, 1,8-cineole, linalool, α-terpineol | Herbarium CAG 1086 | [91] | |
| Santolina corsica | Essential oil | Aerial parts | Camphor, artemisia ketone, borneol, aromadendrene, muurolene | Herbarium CAG 732/A | [233] |
| Essential oil * | Leaves | Acyclic sesquiterpenes aldehydes (3,9-dimethyl-6-isopropyl-2(E),7(E),9-decatrienal ** and 3,9-dimethyl-6-isopropyl-2(Z),7(E),9-decatrienal) ** | N.A. | [234] | |
| Essential oil * | Aerial parts | Artemisia ketone, β-phellandrene, myrcene, santolina triene, 1,8-cineole, β-pinene, isolyratol | N.A. | [67] | |
| Essential oil * | Aerial parts | Myrcene, santolina triene, β-phellandrene, lyratol, β-pinene, sabinene | N.A. | [68,69] | |
| Essential oil | Aerial parts | Three chemotypes (artemisia ketone-β-phellandrene; myrcene; β-phellandrene-myrcene) | Herbarium SASSA 732 | [151] | |
| Diethyl ether extract * | Roots | Monoterpenes (menthol); sesquiterpene hydrocarbons (β-sesquiphellandrene, α- and β-selinenes, β-elemene); terpenes and acetylene derivatives: (Z)-furylthienylbutenyne, (E)-furylthienylbutenyne, dammaradyenil acetate, friedeline, (Z)-acetoxymethylfurylthienylbutenyne, (E)-acetoxymethylfurylthienylbutenyne, 3-Epifriedelinol, dammaradienol, spiroketalenol ether, selin-11-en-4α-ol | N.A. | [235] | |
| Methanol extract * | Aerial parts | Kaempferol-3-O-glucoside, chlorogenic acid, rosmarinic acid, genistin, caffeic acid, ferulic acid, quercetin-3-O-glucoside, vanillic acid, protocatechuic acid, (-)-epicatechin, gallic acid, neochlorogenic acid | 011151 and 011152 | [110] | |
| N-hexane extract * | Aerial parts | Myrcene, palmitic acid methyl ester, palmitic acid ethyl ester, β-phellandrene, ar-curcumene, α-amyrin, β-amyrin, 1,8-cineole | 011151 and 011152 | [110] | |
| Santolina insularis | Essential oil | Aerial parts | Artemisia ketone, 10-H-cyclopropyl-1,1,7-trimethyl-4-methylen-decahydro azulene, 1,8-cineole, camphene, bornyl acetate, borneol, α-pinene | Herbarium CAG 732 [233] | [93,233] |
| Super critical fluid extract | Leaves | β-myrcene, ar-curcumene, β-phellandrene, trans-β-terpineol, spathulenol, γ-curcumene, 3-thujanol, 1,8-cineole, sabinene; waxes | N.A. | [70] | |
| Hydrodistillated essential oil | Leaves | β-myrcene, β-phellandrene, β-pinene, ar-curcumene, Spathulenol, trans-β-Terpineol | N.A. | [70] | |
| Essential oil | Aerial parts | Four chemotypes (artemisia ketone-β-phellandrene; cis-chrysanthemol-myrcene-β-pinene; β-phellandrene-myrcene; santolina triene) | Herbarium CAG 732 [74] Herbarium CAG 732/b [71] Herbarium SASSA 732 [73] | [71,73,74,189] | |
| Acetone extract | Aerial parts | Eudesmane sesquiterpenoids **, a trans-chrysanthemyl monoterpenoid **, two chrysanthemane monoterpenoids, eucamalol | Herbarium CAG 0707000 | [236] | |
| Acetone extract | Aerial parts | 11 polyoxygenated germacranes (four news **) | Herbarium CAG 732 | [116] | |
| Methanol extract | Leaves | (E)-3-{6-[(E)-3-hydroxy-3-oxo-1-propenyl]-9-oxo-9H-xanthen-2-yl}-2-propenoic acid **, six flavonoids (hispidulin, nepetin, cirsimaritin, rhamnocitrin, luteolin and luteolin 7-O-β-D-glucopyranoside) | Herbarium of the Dipartimento Farmaco Chimico Tecnologico, University of Cagliari (No. 0310) | [152] | |
| Scrophularia trifoliata | Ethanolic extract | Aerial parts | Iridoids: catalpol and aucubin | Herbarium CAG 27-3 | [237] |
| Seseli praecox | Acetone extract | Aerial parts | Coumarins: anomalin, isopterixyn, 3′-angeloyl-(-)-cis-khellactone, bocconin ** | N.A. | [238] |
| Super critical fluid extract | Leaves | Differences between three sites (himachalol, sabinene, β-phellandrene; β-phellandrene, undecane, α-pinene; α-humulene, β-phellandrene, bicyclogermacrene) | N.A. | [195] | |
| Essential oil | Leaves | Differences between three sites (sabinene, β-phellandrene; β-phellandrene, α-pinene; α-humulene, β-phellandrene, bicyclogermacrene) | N.A. | [195] | |
| Stachys corsica | Ethanol extract | Aerial parts | Flavonoid glycosides: isoscutellarein 7-O-(6‴-O-acetyl)-β-D-allopyranosyl-(1‴→2″)-β-D-glucopyranoside and isoscutellarein 4′-methyl ether 7-O-(6‴-O-acetyl)-β-D-allopyranosyl(1‴→2″)-β-D-glucopyranoside; iridoid glucosides: harpagide and acetylharpagide) | Herbarium CAG 29-1 [239] | [239,240] |
| Stachys glutinosa | Acetone and ethanol extracts | Aerial parts | Harpagide, 5-Allosyloxy-aucubin **, acetylharpagide, monomelittoside, melittoside, allobetonicoside | N.A. | [240,241] |
| Essential oil * | Aerial parts | Terpinen-4-ol, α-pinene, α-terpineol, β-phellandrene and γ-terpinene, β-caryophyllene | N.A. | [242] | |
| Essential oil | Aerial parts | Differences between two sites (terpinen 4-ol, α-terpinyl acetate, trans-cadina-1(6),4-diene, α-terpineol; α-cedrene, α-terpineol, terpinen-4-ol, α-terpinyl acetate) | N.A. | [75] | |
| Dichloromethane extract | Aerial parts | Flavones: xanthomicrol, sideritoflavone, 8-methoxycirsilineol and eupatilin; roseostachenone and 3α,4α-epoxyroseostachenol ** | Herbarium of the Department of Life and Environmental Science, Drug Sciences Section (No. 0425) | [186] | |
| Ethanol extract | Aerial parts | Melittoside, caffeoylquinic acid, 3-caffeoylquinic acid, chlorogenic acid, β-OH-forsythoside B, β-OH-acteoside, betonyoside E, hypolaetin-7-O-(2-allosyl)-glucopyranoside, forsythoside B, acteoside, isoscutellarein-7-O-[allosyl(1→2)]-glucopyranoside, isoacteoside, isoscutellarein-7-O-[6‴acetylallosyl-(1→2)]-glucopyranoside, 3′-hydroxy-4″-omethylisoscutellarein-7-O-[6-acetyl-allosyl-(1→2)]-glucopyranoside, 4′-O-methylisoscutellarein-7-O-[allosyl-(1→2)]-glucopyranoside, 3′-hydroxy-4′-O-methylisoscutellarein-7-O-(6‴-acetylhexosyl)-hexoside, isoscutellarein-7-O-[6″,6‴-di-acetyl-allosyl(1→2)]-glucopyranoside, 3′-hydroxy-4′-O-methylisoscutellarein-7-O-[6″,6‴-di-acetylallosyl(1→2)]-glucopyranoside | Herbarium SASSA 1099 | [118] | |
| Tanacetum audibertii | Super critical fluid extract | Aerial parts | Trans-linalyl oxide acetate, artemisia ketone, 1,8-cineole, artemisyl acetate | Herbarium CAG 737/A | [76] |
| Hydrodistillated essential oil | Aerial parts | Artemisia ketone, trans-linalyl oxide acetate and 1,8-cineole, artemisyl acetate | Herbarium CAG 737/A | [76] | |
| Hydroalcoholic extract | Aerial parts | Valine, alanine, aspartic acid, sucrose, α-glucose. Β-glucose, trigonelline, formic acid. Phenolic and flavonoids contents | Herbarium CAG 737/A | [119] | |
| Thymus herba-barona subsp. herba-barona | Diethyl ether extract * | Aerial parts | Flavanones (eriodictyol, naringenin) and flavones (luteolin, sorbifolin, thymusin, cirsiliol, apigenin, sideritoflavone, cirsimaritin, cirsilineol, xanthomicrol, 8-methoxycirsilineol and genkwanin) | N.A. | [243] |
| Essential oil * | Aerial parts | Eight chemotypes (thymol, carvacrol, linalool, geraniol, α-terpenyl acetate, terpinen-4-ol, carvone and cis-dihydrocarvone) | N.A. | [67,190] | |
| Essential oil | Aerial parts | Differences between two sites (thymol, p-cymene, γ-terpinene, linalool; thymol, carvacrol, p-cymene, γ-terpinene borneol) | N.A. | [78] | |
| Essential oil | Aerial parts | Carvacrol, borneol, p-cymene | N.A. | [79] | |
| Essential oil | Aerial parts | Linalool, carvacrol | Herbarium CAG 1065 | [244] | |
| Essential oil | Aerial parts | Carvacrol, thymol | Herbarium CAG 1065 | [77] | |
| Verbascum conocarpum subsp. conocarpum | Ethanolic extract | Aerial parts | One iridoid (aucubin) and a phenyl-propanoid compound (verbascoside) | Herbarium CAG 27-2 | [237] |
| Vinca difformis subsp. sardoa | N.A. | Roots | Alkaloids (norfluorocurarine, akuammigine, carapanaubine, majdine, isomajdine, rauvoxinine, ent-N(1)-methyl-14,15-didehydroaspidospermidine **, N(1)-methyl-14,15-didehydroaspidofractinine **, N(1)-methylaspidofractinine **, N(1)-methyl-14,15-didehydrotuboxenine **) | N.A. | [245] |
| Aqueous acetic acid extract | Aerial parts | Indole alkaloids (conoflorine, N(1)-methyl-14,15-didehydro-12-hydroxyaspidofractinine **, N(1)-methyl-14,15-didehydro-12-methoxyaspidofractinine **, N(1)-formyl-14,15-didehydroaspidofractinine **, N(1)-formyl-14,15-didehydro-12-hydroxyaspidofractinine **, venalstonine and N(1)-methyl-14,15-didehydroaspidofractinine) | N.A. | [246] | |
| Ethanol extract | Aerial parts | Iridoid glucosides (loganic acid and loganin) | N.A. | [247] | |
| Methanolic extract | Leaves | Quinic acid, chlorogenic acid, caffeoylquinic acid isomer 1 and robinin | Herbarium SASSA 820 | [140] | |
| Histochemical preparation | Roots, stems, petioles, leaves and flowers | Indole alkaloids | Herbarium CAG 1301 [248] | [248,249] |
3.4. Criticism and Future Perspectives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kinghorn, A.D.; Pan, L.; Fletcher, J.N.; Chai, H. The relevance of higher plants in lead compound discovery programs. J. Nat. Prod. 2011, 74, 1539–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Li, J.W.-H.; Vederas, J.C. Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Verpoorte, R. Pharmacognosy in the New Millennium: Leadfinding and Biotechnology. J. Pharm. Pharmacol. 2000, 52, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, F. Antimicrobial, antibiotic potentiating activity and phytochemical profile of essential oils from exotic and endemic medicinal plants of Mauritius. Ind. Crops Prod. 2015, 71, 197–204. [Google Scholar] [CrossRef]
- Neergheen, V.S.; Soobrattee, M.A.; Bahorun, T.; Aruoma, O.I. Characterization of the phenolic constituents in Mauritian endemic plants as determinants of their antioxidant activities in vitro. J. Plant Physiol. 2006, 163, 787–799. [Google Scholar] [CrossRef]
- Neergheen, V.S.; Bahorun, T.; Jen, L.S.; Aruoma, O.I. Bioefficacy of mauritian endemic medicinal plants: Assessment of their phenolic contents and antioxidant potential. Pharm. Biol. 2007, 45, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Rangasamy, O.; Mahomoodally, F.M.; Gurib-Fakim, A.; Quetin-Leclercq, J. Two anti-staphylococcal triterpenoid acids isolated from Psiloxylon mauritianum (Bouton ex Hook.f.) Baillon, an endemic traditional medicinal plant of Mauritius. S. Afr. J. Bot. 2014, 93, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Soobrattee, M.A.; Bahorun, T.; Neergheen, V.S.; Googoolye, K.; Aruoma, O.I. Assessment of the content of phenolics and antioxidant actions of the Rubiaceae, Ebenaceae, Celastraceae, Erythroxylaceae and Sterculaceae families of Mauritian endemic plants. Toxicol. Vitr. 2008, 22, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Mittermeier, R.A.; Robles Gil, P.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; da Fonseca, G.A. Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Médail, F. The specific vulnerability of plant biodiversity and vegetation on Mediterranean islands in the face of global change. Reg. Environ. Chang. 2017, 17, 1775–1790. [Google Scholar] [CrossRef] [Green Version]
- Fenu, G.; Bacchetta, G.; Christodoulou, C.S.; Cogoni, D.; Fournaraki, C.; del Galdo Gian Pietro, G.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; et al. A common approach to the conservation of threatened island vascular plants: First results in the mediterranean basin. Diversity 2020, 12, 157. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D. Plant Evolution in the Mediterranean; Oxford University Press: Oxford, UK, 2005; ISBN 0198515332. [Google Scholar]
- Fenu, G.; Fois, M.; Cañadas, E.M.; Bacchetta, G. Using endemic-plant distribution, geology and geomorphology in biogeography: The case of Sardinia (mediterranean basin). Syst. Biodivers. 2014, 12, 181–193. [Google Scholar] [CrossRef]
- Fenu, G.; Giusso Del Galdo, G.; Montmollin De, B.; Gotsiou, P.; Cogoni, D.; Piazza, C.; Fournaraki, C.; Kyratzis, A.C.; Vicens, M.; Christodoulou, C.S.; et al. Active management actions for the conservation of the endangered Mediterranean island flora: The CARE-MEDIFLORA project. Plant Sociol. 2017, 54, 101–110. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Lussu, M.; Marignani, M.; Lai, R.; Loi, M.C.; Cogoni, A.; Cortis, P. A Synopsis of Sardinian Studies: Why Is It Important to Work on Island Orchids? Plants 2020, 9, 853. [Google Scholar] [CrossRef]
- Bacchetta, G.; Fenu, G.; Guarino, R.; Mandis, G.; Mattana, E.; Nieddu, G.; Scudu, C. Floristic traits and biogeographic characterization of the Gennargentu massif (Sardinia). Candollea 2013, 68, 209–220. [Google Scholar] [CrossRef]
- Fenu, G.; Mattana, E.; Congiu, A.; Bacchetta, G. The Endemic Vascular Flora of Supramontes (Sardinia), a Priority Plant Conservation Area. Candollea 2010, 65, 347–358. [Google Scholar] [CrossRef]
- Sanna, C.; Ballero, M.; Frailis, L. Bibliografia etnobotanica italiana. Rend. Semin. Fac. Sci. Univ. Cagliari 2007, 77, 31–70. [Google Scholar]
- Leonti, M.; Casu, L.; Sanna, F.; Bonsignore, L. A comparison of medicinal plant use in Sardinia and Sicily-De Materia Medica revisited? J. Ethnopharmacol. 2009, 121, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Stepp, J.R.; Moerman, D.E. The importance of weeds in ethnopharmacology. J. Ethnopharmacol. 2001, 75, 19–23. [Google Scholar] [CrossRef]
- Loi, M.; Frailis, L.; Maxia, A. Le piante utilizzate nella medicina popolare nel territorio di Gesturi (Sardegna centro-meridionale). Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2002, 109, 167–176. [Google Scholar]
- Ballero, M.; Poli, F. Plants used in folk medicine of Monteleone (northern Sardinia). Fitoterapia 1998, 69, 52–64. [Google Scholar]
- Ballero, M.; Floris, R.; Poli, F. Le piante utilizzate nella medicina popolare nel territorio di Laconi (Sardegna centrale). Boll. Soc. Sarda Sci. Nat. 1997, 31, 207–229. [Google Scholar]
- Atzei, A.D. Le Piante Nella Tradizione Popolare Della Sardegna; Carlo Delfino Editore: Sassari, Italy, 2003. [Google Scholar]
- Fabricant, D.S.; Farnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109, 69–75. [Google Scholar] [CrossRef]
- Ballero, M.; Fresu, I. Le piante di uso officinale nella Barbagia di Seui (Sardegna centrale). Fitoterapia 1993, 64, 141–150. [Google Scholar]
- Sanna, C.; Ballero, M.; Maxia, A. Le piante medicinali utilizzate contro le patologie epidermiche in Ogliastra (Sardegna centro-orientale). Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2006, 113, 73–82. [Google Scholar]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Loi, M.C.; Poli, F.; Sacchetti, G.; Selenu, M.B.; Ballero, M. Ethnopharmacology of Ogliastra (Villagrande Strisaili, Sardinia, Italy). Fitoterapia 2004, 75, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Ballero, M.; Bruni, A.; Sacchetti, G.; Poli, F. Le piante utilizzate nella medicina popolare nel comune di Tempio Pausania (Sardegna settentrionale). Acta Phyther. 1997, 2, 23–29. [Google Scholar]
- Ballero, M.; Poli, F.; Sacchetti, G.; Loi, M.C. Ethnobotanical research in the territory of Fluminimaggiore (south-western Sardinia). Fitoterapia 2001, 72, 788–801. [Google Scholar] [CrossRef]
- Loi, M.C.; Maxia, L.; Maxia, A. Ethnobotanical comparison between the villages of Escolca and Lotzorai (Sardinia, Italy). J. Herbs Spices Med. Plants 2005, 11, 67–84. [Google Scholar] [CrossRef]
- Ballero, M.; Bruni, A.; Sacchetti, G.; Poli, F. Indagine etnofarmacobotanica del territorio di Arzana (Sardegna orientale). Ann. Bot. 1994, 52, 489–500. [Google Scholar]
- Ballero, M.; Fresu, I. Le piante officinali impiegate nel territorio del Marganai (Sardegna sud-occidentale). Fitoterapia 1991, 62, 524–531. [Google Scholar]
- Ballero, M.; Sacchetti, G.; Poli, F. Plants in folk medicine in the territory of Perdasdefogu (Central Sardinia, Italy). Allionia 1997, 35, 157–164. [Google Scholar]
- Ballero, M.; Floris, R.; Sacchetti, G.; Poli, F. Ricerche etnobotaniche nel comune di Ussassai (Sardegna Centro-Orientale). Atti Della Soc. Toscana Sci. Nat. Mem. Ser. B 1998, 105, 83–87. [Google Scholar]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. Wild Food Thistle Gathering and Pastoralism: An Inextricable Link in the Wild Food Thistle Gathering and Pastoralism: An Inextricable Link in the Biocultural Landscape of Barbagia, Central Sardinia (Italy). Sustainability 2020, 12, 5105. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance and Primary Health Care; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Petretto, G.L.; Chessa, M.; Piana, A.; Masia, M.D.; Foddai, M.; Mangano, G.; Culeddu, N.; Afifi, F.U.; Pintore, G. Chemical and biological study on the essential oil of Artemisia caerulescens L. ssp. densiflora (Viv.). Nat. Prod. Res. 2013, 27, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Chessa, M.; Sias, A.; Piana, A.; Mangano, G.S.; Petretto, G.L.; Masia, M.D.; Tirillini, B.; Pintore, G. Chemical composition and antibacterial activity of the essential oil from Mentha requienii Bentham. Nat. Prod. Res. 2013, 27, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Bertoli, A.; Lepori, E.; Morelli, I.; Panizzi, L. Antimicrobial and antifungal activity of crude extracts and isolated saponins from Astragalus verrucosus. Fitoterapia 2002, 73, 336–339. [Google Scholar] [CrossRef]
- Aboody, M.S.A.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [Green Version]
- Esposito, M.; Nim, S.; Nothias, L.F.; Gallard, J.F.; Rawal, M.K.; Costa, J.; Roussi, F.; Prasad, R.; Di Pietro, A.; Paolini, J.; et al. Evaluation of Jatrophane Esters from Euphorbia spp. as Modulators of Candida albicans Multidrug Transporters. J. Nat. Prod. 2017, 80, 479–487. [Google Scholar] [CrossRef]
- Usai, M.; Juliano, C.; Pintore, G.; Chessa, M. Preliminarly study of composition and antimicrobial activity of essential oil of Glechoma sardoa Bég. Acta Hortic. 2004, 597, 125–128. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Arlorio, M.; Coisson, J.D.; Russo, M.T.; Pirisi, F.M.; Satta, M.; Cabras, P. Chemical composition, plant genetic differences, and antifungal activity of the essential oil of Helichrysum italicum G. Don ssp. microphyllum (Willd) Nym. J. Agric. Food Chem. 2003, 51, 1030–1034. [Google Scholar] [CrossRef]
- Juliano, C.; Marchetti, M.; Campagna, P.; Usai, M. Antimicrobial activity and chemical composition of essential oil from Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso collected in South-West Sardinia. Saudi J. Biol. Sci. 2019, 26, 897–905. [Google Scholar] [CrossRef]
- Tundis, R.; Statti, G.A.; Conforti, F.; Bianchi, A.; Agrimonti, C.; Sacchetti, G.; Muzzoli, M.; Ballero, M.; Menichini, F.; Poli, F. Influence of environmental factors on composition of volatile constituents and biological activity of Helichrysum italicum (Roth) Don (Asteraceae). Nat. Prod. Res. 2005, 19, 379–387. [Google Scholar] [CrossRef]
- Chinou, I.B.; Roussis, V.; Perdetzoglou, D.; Loukis, A. Chemical and biological studies on two Helichrysum species of Greek origin. Planta Med. 1996, 62, 377–379. [Google Scholar] [CrossRef]
- Roussis, V.; Tsoukatou, M.; Chinou, I.B.; Ortiz, A. Composition and Antibacterial Activity of Two Helichrysum Species of Greek Origin. Planta Med. 1997, 63, 181–183. [Google Scholar]
- Taglialatela-Scafati, O.; Pollastro, F.; Chianese, G.; Minassi, A.; Gibbons, S.; Arunotayanun, W.; Mabebie, B.; Ballero, M.; Appendino, G. Antimicrobial phenolics and unusual glycerides from Helichrysum italicum subsp. microphyllum. J. Nat. Prod. 2013, 76, 346–353. [Google Scholar] [CrossRef]
- Marshall, N.J.; Piddock, L.J. Antibacterial efflux systems. Microbiologia 1997, 13, 285–300. [Google Scholar] [PubMed]
- Donadu, M.G.; Usai, D.; Marchetti, M.; Usai, M.; Mazzarello, V.; Molicotti, P.; Montesu, M.A.; Delogu, G.; Zanetti, S. Antifungal activity of oils macerates of North Sardinia plants against Candida species isolated from clinical patients with candidiasis. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Mandrone, M.; Bonvicini, F.; Lianza, M.; Sanna, C.; Maxia, A.; Gentilomi, G.A.; Poli, F. Sardinian plants with antimicrobial potential. Biological screening with multivariate data treatment of thirty-six extracts. Ind. Crops Prod. 2019, 137, 557–565. [Google Scholar] [CrossRef]
- Casu, L.; Cottiglia, F.; Leonti, M.; De Logu, A.; Agus, E.; Tse-Dinh, Y.C.; Lombardo, V.; Sissi, C. Ungeremine effectively targets mammalian as well as bacterial type i and type II topoisomerases. Bioorg. Med. Chem. Lett. 2011, 21, 7041–7044. [Google Scholar] [CrossRef] [Green Version]
- Bonvicini, F.; Antognoni, F.; Iannello, C.; Maxia, A.; Poli, F.; Gentilomi, G.A. Relevant and selective activity of Pancratium illyricum L. against Candida albicans clinical isolates: A combined effect on yeast growth and virulence. BMC Complement. Altern. Med. 2014, 14, 409. [Google Scholar] [CrossRef] [Green Version]
- Ghosal, S.; Singh, S.K.S.; Kumar, Y.; Unnikrishnan, S.; Chattopadhyay, S. The role of ungeremine in the growth-inhibiting and cytotoxic effects of lycorine: Evidence and speculation. Planta Med. 1988, 54, 114–116. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E.; Laconi, S.; Deidda, D.; Maxia, A. Chemical and biological comparisons on supercritical extracts of Tanacetum cinerariifolium (Trevir) Sch. Bip. with three related species of chrysanthemums of Sardinia (Italy). Nat. Prod. Res. 2009, 23, 190–199. [Google Scholar] [CrossRef]
- Soković, M.D.; Brkić, D.D.; Džamić, A.M.; Ristić, M.S.; Marin, P.D. Chemical composition and antifungal activity of Salvia desoleana Atzei & Picci essential oil and its major components. Flavour Fragr. J. 2009, 24, 83–87. [Google Scholar] [CrossRef]
- Peana, A.T.; Moretti, M.D.L.; Juliano, C. Chemical composition and antimicrobial action of the essential oils of Salvia desoleana and S. sclarea. Planta Med. 1999, 65, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J.; Muselli, A.; Serra, D.D.R.; Gonny, M.; Bolla, J.M. Antibacterial action of essential oils from Corsica. J. Essent. Oil Res. 2007, 19, 176–182. [Google Scholar] [CrossRef]
- Liu, K.; Rossi, P.-G.; Ferrari, B.; Berti, L.; Casanova, J.; Tomi, F. Composition, irregular terpenoids, chemical variability and antibacterial activity of the essential oil from Santolina corsica Jordan et Fourr. Phytochemistry 2007, 68, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Guinoiseau, E.; Luciani, A.; Rossi, P.G.; Quilichini, Y.; Ternengo, S.; Bradesi, P.; Berti, L. Cellular effects induced by Inula graveolens and Santolina corsica essential oils on Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Cherchi, G.; Deidda, D.; Gioannis, B.D.; Marongiu, B.; Pompei, R.; Porcedda, S. Extraction of Santolina insularis essential oil by supercritical carbon dioxide: Influence of some process parameters and biological activity. Flavour Fragr. J. 2001, 16, 35–43. [Google Scholar] [CrossRef]
- Alves-Silva, J.M.; Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.J.; Cruz, M.T.; Salgueiro, L. Chemical characterization and bioactivity of the essential oil from Santolina insularis, a Sardinian endemism. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- Radulović, N.S.; Randjelović, P.J.; Stojanović, N.M.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Ilić, I.R.; Djordjević, V.B. Toxic essential oils. Part II: Chemical, toxicological, pharmacological and microbiological profiles of Artemisia annua L. volatiles. Food Chem. Toxicol. 2013, 58, 37–49. [Google Scholar] [CrossRef]
- Juliano, C.; Marchetti, M.; Pisu, M.L.; Usai, M. In vitro antimicrobial activity of essential oils from Sardinian flora against Cutibacterium (Formerly propionibacterium) acnes and its enhancement by chitosan. Sci. Pharm. 2018, 86, 40. [Google Scholar] [CrossRef] [Green Version]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Maxia, A.; Murgia, S.; Pons, R.; Demurtas, D.; Pando, D.; Falconieri, D.; Peris, J.E.; et al. Faceted phospholipid vesicles tailored for the delivery of Santolina insularis essential oil to the skin. Colloids Surf. B Biointerfaces 2015, 132, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Pintore, G.; Chessa, M.; Manconi, P.; Zanetti, S.; Deriu, A.; Tirillini, B. Chemical composition and antimicrobial activities of essential oil of Stachys glutinosa from Sardinia. Nat. Prod. Commun. 2006, 1, 1133–1136. [Google Scholar]
- Maxia, A.; Sanna, C.; Piras, A.; Porcedda, S.; Falconieri, D.; Gonçalves, M.J.M.J.; Cavaleiro, C.; Salgueiro, L. Chemical composition and biological activity of Tanacetum audibertii (Req.) DC. (Asteraceae), an endemic species of Sardinia Island, Italy. Ind. Crops Prod. 2015, 65, 472–476. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Benzarti, A.; Marongiu, B.; Maxia, A.; Piras, A.; Salgueiro, L. Antifungal and anti-inflammatory potential of Lavandula stoechas and Thymus herba-barona essential oils. Ind. Crops Prod. 2013, 44, 97–103. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Juliano, C.; Mattana, A.; Usai, M. Composition and in vitro antimicrobial activity of the essential oil of Thymus herba-barona Loisel growing wild in Sardinia. J. Essent. Oil Res. 2000, 12, 516–522. [Google Scholar] [CrossRef]
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral potentials of medicinal plants. Virus Res. 2008, 131, 111–120. [Google Scholar] [CrossRef]
- Nothias, L.F.; Boutet-Mercey, S.; Cachet, X.; De La Torre, E.; Laboureur, L.; Gallard, J.F.; Retailleau, P.; Brunelle, A.; Dorrestein, P.C.; Costa, J.; et al. Environmentally Friendly Procedure Based on Supercritical Fluid Chromatography and Tandem Mass Spectrometry Molecular Networking for the Discovery of Potent Antiviral Compounds from Euphorbia semiperfoliata. J. Nat. Prod. 2017, 80, 2620–2629. [Google Scholar] [CrossRef]
- Nothias-Scaglia, L.-F.; Retailleau, P.; Paolini, J.; Pannecouque, C.; Neyts, J.; Dumontet, V.; Roussi, F.; Leyssen, P.; Costa, J.; Litaudon, M. Jatrophane diterpenes as inhibitors of chikungunya virus replication: Structure-activity relationship and discovery of a potent lead. J. Nat. Prod. 2014, 77, 1505–1512. [Google Scholar] [CrossRef]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol α-pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef]
- Esposito, F.; Sanna, C.; Del Vecchio, C.; Cannas, V.; Venditti, A.; Corona, A.; Bianco, A.; Serrilli, A.M.; Guarcini, L.; Parolin, C.; et al. Hypericum hircinum L. Components as new single-molecule inhibitors of both HIV-1 reverse transcriptase-associated DNA polymerase and ribonuclease H activities. Pathog. Dis. 2013, 68, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Bailas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-aids agents, 11 betulinic acid and platanic acid as anti-HIV principles from syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Scognamiglio, M.; Fiorentino, A.; Corona, A.; Graziani, V.; Caredda, A.; Cortis, P.; Montisci, M.; Ceresola, E.R.; Canducci, F.; et al. Prenylated phloroglucinols from Hypericum scruglii, an endemic species of Sardinia (Italy), as new dual HIV-1 inhibitors effective on HIV-1 replication. PLoS ONE 2018, 12, e0195168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridi, H.; De Carvalho Meirelle, G.; von Poser, G.L. Structural diversity and biological activities of phloroglucinol derivatives from Hypericum species. Phytochemistry 2018, 155, 203–232. [Google Scholar] [CrossRef]
- Sanna, C.; Rigano, D.; Corona, A.; Piano, D.; Formisano, C.; Farci, D.; Franzini, G.; Ballero, M.; Chianese, G.; Tramontano, E.; et al. Dual HIV-1 reverse transcriptase and integrase inhibitors from Limonium morisianum Arrigoni, an endemic species of Sardinia (Italy). Nat. Prod. Res. 2019, 33, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Daino, G.L.; Frau, A.; Sanna, C.; Rigano, D.; Distinto, S.; Madau, V.; Esposito, F.; Fanunza, E.; Bianco, G.; Taglialatela-Scafati, O.; et al. Identification of Myricetin as an Ebola Virus VP35-Double-Stranded RNA Interaction Inhibitor through a Novel Fluorescence-Based Assay. Biochemistry 2018, 57, 6367–6378. [Google Scholar] [CrossRef] [Green Version]
- Cagno, V.; Sgorbini, B.; Sanna, C.; Cagliero, C.; Ballero, M.; Civra, A.; Donalisio, M.; Bicchi, C.; Lembo, D.; Rubiolo, P. In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei & V. Picci essential oil. PLoS ONE 2017, 12, e0172322. [Google Scholar] [CrossRef] [Green Version]
- De Logu, A.; Loy, G.; Pellerano, M.L.; Bonsignore, L.; Schivo, M.L. Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularis essential oil. Antivir. Res. 2000, 48, 177–185. [Google Scholar] [CrossRef]
- Valenti, D.; De Logu, A.; Loy, G.; Sinico, C.; Bonsignore, L.; Cottiglia, F.; Garau, D.; Fadda, A. Liposome-incorporated Santolina insularis essential oil: Preparation, characterization and in vitro antiviral activity. J. Liposome Res. 2001, 11, 73–90. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on Cancer: Setting Priorities Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020; ISBN 9789240001299. [Google Scholar]
- Shin, S.-A.; Moon, S.Y.; Kim, W.-Y.; Paek, S.-M.; Park, H.H.; Lee, C.S. Structure-Based Classification and Anti-Cancer Effects of Plant Metabolites. Int. J. Mol. Sci. 2018, 19, 2651. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, G.; Carcache, P.J.B.; Addo, E.M.; Kinghorn, A.D. Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol. Adv. 2020, 38, 107337. [Google Scholar] [CrossRef] [PubMed]
- Ornano, L.; Venditti, A.; Sanna, C.; Ballero, M.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Bianco, A. Chemical composition and biological activity of the essential oil from Helichrysum microphyllum cambess. ssp. tyrrhenicum Bacch., Brullo e Giusso growing in La Maddalena archipelago, Sardinia. J. Oleo Sci. 2015, 64, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Ornano, L.; Venditti, A.; Ballero, M.; Sanna, C.; Donno, Y.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Tirillini, B.; et al. Essential oil composition and biological activity from Artemisia caerulescens subsp. densiflora (Viv.) Gamisans ex Kerguélen & Lambinon (Asteraceae), an endemic species in the habitat of La Maddalena Archipelago. Nat. Prod. Res. 2016, 30, 1802–1809. [Google Scholar] [CrossRef]
- Calcabrini, A.; Stringaro, A.; Toccacieli, L.; Meschini, S.; Marra, M.; Colone, M.; Salvatore, G.; Mondello, F.; Arancia, G.; Molinari, A. Terpinen-4-ol, the Main Component of Melaleuca alternifolia (Tea Tree) Oil Inhibits the in Vitro Growth of Human Melanoma Cells. J. Investig. Dermatol. 2004, 122, 349–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greay, S.J.; Ireland, D.J.; Kissick, H.T.; Levy, A.; Beilharz, M.W.; Riley, T.V.; Carson, C.F. Induction of necrosis and cell cycle arrest in murine cancer cell lines by Melaleuca alternifolia (tea tree) oil and terpinen-4-ol. Cancer Chemother. Pharmacol. 2010, 65, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Atzeri, A.; Nieddu, M.; Appendino, G. New insights into the antioxidant activity and cytotoxicity of arzanol and effect of methylation on its biological properties. Chem. Phys. Lipids 2017, 205, 55–64. [Google Scholar] [CrossRef]
- Cottiglia, F.; Casu, L.; Bonsignore, L.; Casu, M.; Floris, C.; Leonti, M.; Gertsch, J.; Heilmann, J. New cytotoxic prenylated isoflavonoids from Bituminaria morisiana. Planta Med. 2005, 71, 254–260. [Google Scholar] [CrossRef]
- Leonti, M.; Casu, L.; Gertsch, J.; Bonsignore, L.; Floris, C.; Casu, M.; Cottiglia, F. A pterocarpan from the seeds of Bituminaria morisiana. J. Nat. Med. 2010, 64, 354–357. [Google Scholar] [CrossRef]
- Miglietta, A.; Gabriel, L.; Appendino, G.; Bocca, C. Biological properties of jatrophane polyesters, new microtubule-interacting agents. Cancer Chemother. Pharmacol. 2003, 51, 67–74. [Google Scholar] [CrossRef]
- Poli, F.; Appendino, G.; Sacchetti, G.; Ballero, M.; Maggiano, N.; Ranelletti, F.O. Antiproliferative effects of daucane esters from Ferula communis and F. arrigonii on human colon cancer cell lines. Phyther. Res. 2005, 19, 152–157. [Google Scholar] [CrossRef]
- Appendino, G.; Jakupovic, J.; Alloatti, S.; Ballero, M. Daucane esters from Ferula arrigonii. Phytochemistry 1997, 45, 1639–1643. [Google Scholar] [CrossRef]
- Ranelletti, F.O.; Ricci, R.; Larocca, L.M.; Maggiano, N.; Capelli, A.; Scambia, G.; Benedetti-Panici, P.; Mancuso, S.; Rumi, C.; Piantelli, M. Growth-inhibitory effect of quercetin and presence of type-II estrogen-binding sites in human colon-cancer cell lines and primary colorectal tumors. Int. J. Cancer 1992, 50, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.G.; Evans-roberts, K.; Maxwell, A. Macromolecules DNA Topoisomerases. EcoSal Plus 2015, 6. [Google Scholar] [CrossRef]
- Casu, L.; Bonsignore, L.; Pinna, M.; Casu, M.; Floris, C.; Gertsch, J.; Cottiglia, F. Cytotoxic diacetylenic spiroketal enol ethers from Plagius flosculosus. J. Nat. Prod. 2006, 69, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Brindisi, M.; Armentano, B.; Curcio, R.; Sicari, V.; Loizzo, M.R.; Cappello, M.S.; Bedini, G.; Peruzzi, L.; Tundis, R. Exploring the anti-proliferative, pro-apoptotic, and antioxidant properties of Santolina corsica Jord. & Fourr. (Asteraceae). Biomed. Pharmacother. 2018, 107, 967–978. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. α-Pinene Inhibits Human Prostate Cancer Growth in a Mouse Xenograft Model. Chemotherapy 2018, 63, 1–7. [Google Scholar] [CrossRef]
- Manuele, M.G.; Arcos, M.L.B.; Davicino, R.; Ferraro, G.; Cremaschi, G.; Anesini, C. Mechanism of the antiproliferative action of limonene on a lymphoma cell line: Participation of nitric oxide. antiproliferative action of limonene on a lymphoma cell line. Phytother. Res. 2009, 23, 1011–1017. [Google Scholar] [CrossRef]
- García, E.R.; Gutierrez, E.A.; de Melo, F.C.S.A.; Novaes, R.D.; Gonçalves, R.V. Flavonoids Effects on Hepatocellular Carcinoma in Murine Models: A Systematic Review. Evid. Based Complement. Alternat. Med. 2018, 2018, 6328970. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Appendino, G.; Aviello, G.; Ballero, M.; Borrelli, F.; Fattorusso, E.; Petrucci, F.; Santelia, F.U.; Taglialatela-Scafati, O. Cytotoxic germacrane sesquiterpenes from the aerial parts of Santolina insularis. J. Nat. Prod. 2005, 68, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Eakin, M.A.; Thomas, A.M. Tumor Inhibitors. 69. Structure-Cytotoxicity Relationships among the Sesquiterpene Lactones. J. Med. Chem. 1971, 14, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Leporini, L.; Menghini, L.; Foddai, M.; Petretto, G.L.; Chessa, M.; Tirillini, B.; Pintore, G. Antioxidant and antiproliferative activity of Stachys glutinosa L. ethanol extract. Nat. Prod. Res. 2015, 29, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Cappadone, C.; Mandrone, M.; Chiocchio, I.; Sanna, C.; Malucelli, E.; Bassi, V.; Picone, G.; Poli, F. Antitumor potential and phytochemical profile of plants from Sardinia (Italy), a hotspot for biodiversity in the mediterranean basin. Plants 2020, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [Green Version]
- Folkman, J. Fighting cancer by attacking its blood supply. Sci. Am. 1996, 275, 150–154. [Google Scholar] [CrossRef]
- Bhattacharya, S. Natural antimutagens: A review. Res. J. Med. Plant. 2011, 5, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Verschaeve, L. Genotoxicity and antigenotoxicity studies of traditional medicinal plants: How informative and accurate are the results? Nat. Prod. Commun. 2015, 10, 1489–1493. [Google Scholar] [CrossRef]
- Scarpato, R.; Paganucci, L.; Bertoli, A.; Fiore, L.; Pistelli, L.; Federico, G. Licoflavone C attenuates the genotoxicity of cancer drugs in human peripheral lymphocytes. Phyther. Res. 2008, 22, 1650–1654. [Google Scholar] [CrossRef]
- Noel, S.; Kasinathan, M.; Rath, S.K. Evaluation of apigenin using in vitro cytochalasin blocked micronucleus assay. Toxicol. Vitr. 2006, 20, 1168–1172. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.A.; Bowman, A. Oxidative stress and ageing. Br. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, A.; Deiana, M.; Corona, G.; Atzeri, A.; Incani, A.; Appendino, G.; Dessì, M.A. Antioxidant properties of extracts and compounds from Psoralea morisiana. Eur. J. Lipid Sci. Technol. 2005, 107, 521–529. [Google Scholar] [CrossRef]
- Bruni, R.; Muzzoli, M.; Ballero, M.; Loi, M.C.; Fantin, G.; Poli, F.; Sacchetti, G. Tocopherols, fatty acids and sterols in seeds of four Sardinian wild Euphorbia species. Fitoterapia 2004, 75, 50–61. [Google Scholar] [CrossRef]
- Serrilli, A.M.; Graziosi, V.; Ballero, M.; Foddis, C.; Serafini, M.; Poli, F.; Scartezzini, P.; Bianco, A. Endemic Sardinian plants: The case of Genista cadasonensis Valsecchi. Nat. Prod. Res. 2010, 24, 942–947. [Google Scholar] [CrossRef]
- Bauer, J.; Koeberle, A.; Dehm, F.; Pollastro, F.; Appendino, G.; Northoff, H.; Rossi, A.; Sautebin, L.; Werz, O. Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem. Pharmacol. 2011, 81, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Rosa, A.; Deiana, M.; Atzeri, A.; Corona, G.; Incani, A.; Melis, M.P.; Appendino, G.; Dessì, M.A. Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated α-pyrone-phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum. Chem. Biol. Interact. 2007, 165, 117–126. [Google Scholar] [CrossRef]
- Rosa, A.; Pollastro, F.; Atzeri, A.; Appendino, G.; Melis, M.P.; Deiana, M.; Incani, A.; Loru, D.; Dess, M.A. Protective role of arzanol against lipid peroxidation in biological systems. Chem. Phys. Lipids 2011, 164, 24–32. [Google Scholar] [CrossRef]
- Mandrone, M.; Lorenzi, B.; Venditti, A.; Guarcini, L.; Bianco, A.; Sanna, C.; Ballero, M.; Poli, F.; Antognoni, F. Antioxidant and anti-collagenase activity of Hypericum hircinum L. Ind. Crops Prod. 2015, 76, 402–408. [Google Scholar] [CrossRef]
- Mandrone, M.; Scognamiglio, M.; Fiorentino, A.; Sanna, C.; Cornioli, L.; Antognoni, F.; Bonvicini, F.; Poli, F. Phytochemical profile and α-glucosidase inhibitory activity of Sardinian Hypericum scruglii and Hypericum hircinum. Fitoterapia 2017, 120, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Porcu, M.C.; Marongiu, B.; Cossu, A.; Piras, A.; Porcedda, S.; Falconieri, D.; Cappuccinelli, R.; Biosa, G.; Pintus, G.; et al. Antioxidant activity of supercritical carbon dioxide extracts of Salvia desoleana on two human endothelial cell models. Food Res. Int. 2012, 46, 354–359. [Google Scholar] [CrossRef]
- Moulines, J.; Lamidey, A.M.; Desvergnes-Breuil, V. A practical synthesis of ambrox® from sclareol using no metallic oxidant. Synth. Commun. 2001, 31, 749–758. [Google Scholar] [CrossRef]
- Foddai, M.; Maldini, M.; Addis, R.; Petretto, G.L.; Chessa, M.; Pintore, G. Profiling of the bioactive compounds in flowers, leaves and roots of Vinca sardoa. Nat. Prod. Commun. 2017, 12, 933–936. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Marinova, E.M.; Toneva, A.; Yanishlieva, N. Comparison of the antioxidative properties of caffeic and chlorogenic acids. Food Chem. 2009, 114, 1498–1502. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Kishore, N.; Kumar, P.; Shanker, K.; Verma, A.K. Human disorders associated with inflammation and the evolving role of natural products to overcome. Eur. J. Med. Chem. 2019, 179, 272–309. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.; Fairlie, D. Therapeutic Targets in Inflammatory Disease. Curr. Med. Chem. 2005, 12, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Avonto, C.; Rua, D.; Lasonkar, P.B.; Chittiboyina, A.G.; Khan, I.A. Identification of a compound isolated from German chamomile (Matricaria chamomilla) with dermal sensitization potential. Toxicol. Appl. Pharmacol. 2017, 318, 16–22. [Google Scholar] [CrossRef]
- Calzado, M.A.; Lüdi, K.S.; Fiebich, B.; Ben-Neriah, Y.; Bacher, S.; Munoz, E.; Ballero, M.; Prosperini, S.; Appendino, G.; Schmitz, M.L. Inhibition of NF-κB activation and expression of inflammatory mediators by polyacetylene spiroketals from Plagius flosculosus. Biochim. Biophys. Acta Gene Struct. Expr. 2005, 1729, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Peana, A.; Satta, M. A preliminary research on essential oil of Salvia sclarea L. and Salvia desoleana Atzei & Picci. Pharmacol. Res. 1993, 27, 25–26. [Google Scholar]
- Foddai, M.; Marchetti, M.; Ruggero, A.; Juliano, C.; Usai, M. Evaluation of chemical composition and anti-inflammatory, antioxidant, antibacterial activity of essential oil of Sardinian Santolina corsica Jord. & Fourr. Saudi J. Biol. Sci. 2019, 26, 930–937. [Google Scholar] [CrossRef]
- Cottiglia, F.; Casu, L.; Bonsignore, L.; Casu, M.; Floris, C.; Sosa, S.; Altinier, G.; Della Loggia, R. Topical anti-inflammatory activity of flavonoids and a new xanthone from Santolina insularis. Z. Fur Naturforsch. Sect. C J. Biosci. 2005, 60, 63–66. [Google Scholar] [CrossRef]
- Boeing, T.; de Souza, P.; Speca, S.; Somensi, L.B.; Mariano, L.N.B.; Cury, B.J.; Ferreira dos Anjos, M.; Quintão, N.L.M.; Dubuqoy, L.; Desreumax, P.; et al. Luteolin prevents irinotecan-induced intestinal mucositis in mice through antioxidant and anti-inflammatory properties. Br. J. Pharmacol. 2020, 177, 2393–2408. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Harvell, J.D.; Maibach, H.I. Percutaneous absorption and inflammation in aged skin: A review. J. Am. Acad. Dermatol. 1994, 31, 1015–1021. [Google Scholar] [CrossRef]
- Thring, T.S.A.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crops Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.; Zhang, H.; Liu, Z. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phyther. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phyther. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [Green Version]
- Viggiani, M.T.; Polimeno, L.; Di Leo, A.; Barone, M. Phytoestrogens: Dietary intake, bioavailability, and protective mechanisms against colorectal neoproliferative lesions. Nutrients 2019, 11, 1709. [Google Scholar] [CrossRef] [Green Version]
- Garritano, S.; Pinto, B.; Giachi, I.; Pistelli, L.; Reali, D. Assessment of estrogenic activity of flavonoids from Mediterranean plants using an in vitro short-term test. Phytomedicine 2005, 12, 143–147. [Google Scholar] [CrossRef]
- Pinto, B.; Bertoli, A.; Noccioli, C.; Garritano, S.; Reali, D.; Pistelli, L. Estradiol-antagonistic activity of phenolic compounds from leguminous plants. Phytother. Res. 2008, 22, 362–366. [Google Scholar] [CrossRef]
- Zierau, O.; Gester, S.; Schwab, P.; Metz, P.; Kolba, S.; Wulf, M.; Vollmer, G. Estrogenic activity of the phytoestrogens naringenin, 6-(1,1-dimethylallyl) naringenin and 8-prenylnaringenin. Planta Med. 2002, 68, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowarah, J.; Singh, V.P. Anti-diabetic drugs recent approaches and advancements. Bioorg. Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef] [PubMed]
- Bashary, R.; Vyas, M.; Nayak, S.K.; Suttee, A.; Verma, S.; Narang, R.; Khatik, G.L. An Insight of Alpha-amylase Inhibitors as a Valuable Tool in the Management of Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2019, 16, 117–136. [Google Scholar] [CrossRef] [PubMed]
- Hedrington, M.S.; Davis, S.N. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opin. Pharmacother. 2019, 20, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Agatonovic-Kustrin, S.; Kustrin, E.; Gegechkori, V.; Morton, D.W. Bioassay-guided identification of α-amylase inhibitors in herbal extracts. J. Chromatogr. A 2020, 1620, 460970. [Google Scholar] [CrossRef]
- Beidokhti, M.N.; Eid, H.M.; Villavicencio, M.L.S.; Jäger, A.K.; Lobbens, E.S.; Rasoanaivo, P.R.; McNair, L.M.; Haddad, P.S.; Staerk, D. Evaluation of the antidiabetic potential of Psidium guajava L. (Myrtaceae) using assays for α-glucosidase, α-amylase, muscle glucose uptake, liver glucose production and triglyceride accumulation in adipocytes. J. Ethnopharmacol. 2020, 112877. [Google Scholar] [CrossRef]
- Sun, L.; Warren, F.J.; Gidley, M.J. Natural products for glycaemic control: Polyphenols as inhibitors of alpha-amylase. Trends Food Sci. Technol. 2019, 91, 262–273. [Google Scholar] [CrossRef]
- Foddai, M.; Kasabri, V.; Petretto, G.L.; Azara, E.; Sias, A.; Afifi, F.U.; Delogu, G.; Chessa, M.; Pintore, G. In vitro inhibitory effects of Limonium contortirameum and L. virgatum extracts from Sardinia on α-amylase, α-glucosidase and pancreatic lipase. Nat. Prod. Commun. 2014, 9, 181–184. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.A.; Ahmed, Z.A.; Mahwi, T.O.; Aziz, T.A. Effect of quercetin on postprandial glucose excursion after mono- and disaccharides challenge in normal and diabetic rats. J. Diabetes Mellit. 2012, 02, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.-D.; Duan, Y.-Q.; Gao, J.-M.; Ruan, Z.-G. Screening for anti-lipase properties of 37 traditional Chinese medicinal herbs. J. Chin. Med. Assoc. 2010, 73, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Oi, Y.; Hou, I.C.; Fujita, H.; Yazawa, K. Antiobesity effects of Chinese black tea (Pu-erh tea) extract and gallic acid. Phyther. Res. 2012, 26, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Mohan, M.; Kasture, S.; Ballero, M.; Maxia, A.; Sanna, C. Protective effect of Hypericum hircinum on doxorubicin-induced cardiotoxicity in rats. Nat. Prod. Res. 2013, 27. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Ono, K.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Kuwabara, Y.; Nakashima, Y.; Takanabe-Mori, R.; Nishi, E.; et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc. Res. 2010, 87, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, V.S.; Lenihan, D.J.; Ky, B. Cancer therapy-induced cardiotoxicity: Basic mechanisms and potential cardioprotective therapies. J. Am. Heart Assoc. 2014, 3, e000665. [Google Scholar] [CrossRef] [Green Version]
- Carta, M.G.; Manconi, M.; Bacchetta, G.; Orrù, G.; Deidda, M.C.; Musu, M.; Finco, G. Hypericum scruglii Bacchetta, Brullo & Salmeri, is it a possible natural resource against Fibromyalgia? Int. J. Phytomed. 2020, 12, 2. [Google Scholar]
- Chimenti, F.; Cottiglia, F.; Bonsignore, L.; Casu, L.; Casu, M.; Floris, C.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; et al. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: Extraction, biological analysis, and computational study. J. Nat. Prod. 2006, 69, 945–949. [Google Scholar] [CrossRef]
- Wouters, J. Structural aspects of monoamine oxidase and its reversible inhibition. Curr. Med. Chem. 1998, 5, 137–162. [Google Scholar]
- Ceschel, G.C.; Maffei, P.; Moretti, M.D.L.; Demontis, S.; Peana, A.T. In vitro permeation through porcine buccal mucosa of Salvia desoleana Atzei and Picci essential oil from topical formulations. Int. J. Pharm. 2000, 195, 171–177. [Google Scholar] [CrossRef]
- Iannello, C.; Pigni, N.B.; Antognoni, F.; Poli, F.; Maxia, A.; De Andrade, J.P.; Bastida, J. A potent acetylcholinesterase inhibitor from Pancratium illyricum L. Fitoterapia 2014, 92, 163–167. [Google Scholar] [CrossRef]
- Ruiu, S.; Anzani, N.; Orrü, A.; Floris, C.; Caboni, P.; Alcaro, S.; Maccioni, E.; Distinto, S.; Cottiglia, F. Methoxyflavones from Stachys glutinosa with binding affinity to opioid receptors: In silico, in vitro, and in vivo studies. J. Nat. Prod. 2015, 78, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J. The role of the opioid system in alcohol dependence. J. Psychopharmacol. 2014, 28, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Łabuzek, K.; Beil, S.; Beil-Gawełczyk, J.; Gabryel, B.; Franik, G.; Okopień, B. The latest achievements in the pharmacotherapy of gambling disorder. Pharmacol. Rep. 2014, 66, 811–820. [Google Scholar] [CrossRef]
- Gnavi, G.; Bertea, C.M.; Usai, M.; Maffei, M.E. Comparative characterization of Santolina insularis chemotypes by essential oil composition, 5S-rRNA-NTS sequencing and EcoRV RFLP-PCR. Phytochemistry 2010, 71, 930–936. [Google Scholar] [CrossRef]
- Corticchiato, M.; Tomi, F.; François Bernardini, A.; Casanova, J. Composition and infraspecific variability of essential oil from Thymus herba barona Lois. Biochem. Syst. Ecol. 1998, 26, 915–932. [Google Scholar] [CrossRef]
- Biondi, E.; Valentini, G.; Bellomaria, B. Essential Oil of Some Halophyle and Subhalophyle Taxa Artemisia L. from the Central European Mediterranean. J. Essent. Oil Res. 2000, 12, 365–371. [Google Scholar] [CrossRef]
- Usai, M.; Foddai, M.; Bernardini, A.F.; Muselli, A.; Costa, J.; Marchetti, M. Chemical composition and variation of the essential oil of wild sardinian Helichrysum italicum G. Don subsp. microphyllum (Willd.) Nym. from vegetative period to post-blooming. J. Essent. Oil Res. 2010, 22, 373–380. [Google Scholar] [CrossRef]
- Melito, S.; Petretto, G.L.; Podani, J.; Foddai, M.; Maldini, M.; Chessa, M.; Pintore, G. Altitude and climate influence Helichrysum italicum subsp. microphyllum essential oils composition. Ind. Crops Prod. 2016, 80, 242–250. [Google Scholar] [CrossRef]
- Scott, D. Determining the type of relationship between plants and environmental factors. Proc. N. Z. Ecol. Soc. 1969, 16, 29–31. [Google Scholar]
- Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E.; Maxia, A. Isolation of Seseli bocconi Guss, subsp. praecox Gamisans (Apiaceae) volatile oil by supercritical carbon dioxide extraction. Nat. Prod. Res. 2006, 20, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.; Loi, M.C.; Noccioli, C.; Maxia, L.; Moonen, A.C.; Pistelli, L. Volatile constituents as complementary tools to characterize seven sardinian Genista species. Biochem. Syst. Ecol. 2015, 62, 82–90. [Google Scholar] [CrossRef]
- Appendino, G.; Belloro, E.; Tron, G.C.; Jakupovic, J.; Ballero, M. Diterpenoids from Euphorbia pithyusa subsp. cupanii. J. Nat. Prod. 1999, 62, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Jakupovic, S.; Tron, G.C.; Jakupovic, J.; Milon, V.; Ballero, M. Macrocyclic diterpenoids from Euphorbia semiperfoliata. J. Nat. Prod. 1998, 61, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Nothias-Scaglia, L.F.; Gallard, J.F.; Dumontet, V.; Roussi, F.; Costa, J.; Iorga, B.I.; Paolini, J.; Litaudon, M. Advanced Structural Determination of Diterpene Esters Using Molecular Modeling and NMR Spectroscopy. J. Nat. Prod. 2015, 78, 2423–2431. [Google Scholar] [CrossRef]
- Venditti, A.; Lattanzi, C.; Ornano, L.; Maggi, F.; Sanna, C.; Ballero, M.; Alvino, A.; Serafini, M.; Bianco, A. A new glucosidic phthalide from Helichrysum microphyllum subsp. tyrrhenicum from la Maddalena Island (Sardinia, Italy). Nat. Prod. Res. 2016, 30, 789–795. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Donno, Y.; Sanna, C.; Ballero, M.; Bianco, A. Phytochemical analysis of non-volatile fraction of Artemisia caerulescens subsp. densiflora (Viv.) (Asteraceae), an endemic species of la Maddalena Archipelago (Sardinia-Italy). Nat. Prod. Res. 2016, 30, 920–925. [Google Scholar] [CrossRef]
- Pistelli, L.; Pardossi, S.; Flamini, G.; Bertoli, A.; Manunta, A. Three cycloastragenol glucosides from Astragalus verrucosus. Phytochemistry 1997, 45, 585–587. [Google Scholar] [CrossRef]
- Pistelli, L.; Pardossi, S.; Bertoli, A.; Potenza, D. Cicloastragenol glycosides from Astragalus verrucosus. Phytochemistry 1998, 49, 2467–2471. [Google Scholar] [CrossRef]
- Pistelli, L.; Giachi, I.; Lepori, E.; Bertoli, A. Further saponins and flavonoids from Astragalus verrucosus Moris. Pharm. Biol. 2003, 41, 568–572. [Google Scholar] [CrossRef] [Green Version]
- Pistelli, L.; Noccioli, C.; Appendino, G.; Bianchi, F.; Sterner, O.; Ballero, M. Pterocarpans from Bituminaria morisiana and Bituminaria bituminosa. Phytochemistry 2003, 64, 595–598. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Urrestarazu, M.; Rincón-Cervera, M.Á. Sardinian Boraginaceae are new potential sources of gamma-linolenic acid. Food Chem. 2017, 218, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Bulleri, C.; Morelli, I.; Manunta, A. A new flavonoid glycoside from Centaurea horrida. J. Nat. Prod. 2000, 63, 662–663. [Google Scholar] [CrossRef] [PubMed]
- Flamini, G.; Bulleri, C.; Morelli, I. Secondary constituents from Centaurea horrida and their evolutionary meaning. Biochem. Syst. Ecol. 2002, 30, 1051–1054. [Google Scholar] [CrossRef]
- Serafini, M.; Foddai, S.; Ballero, M.; Guiso, M.; Bianco, A. The occurrence of iridoid glycosides in Cymbalaria spp. in Italy. Nat. Prod. Res. 2004, 18, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Spagliardi, P.; Ballero, M.; Seu, G. Macrocyclic diterpenoids from Euphorbia hyberna L. subsp. insularis and their reaction with oxyphilic reagents. Fitoterapia 2002, 73, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Petitto, V.; Serafini, M.; Ballero, M.; Foddai, S.; Stanzione, A.; Nicoletti, M. Iridoids from Euphrasia genargentea, a rare Sardinian endemism. Nat. Prod. Res. 2009, 23, 431–435. [Google Scholar] [CrossRef]
- Casinovi, C.; Tomassini, L.; Nicoletti, M. A new guaianolide from Ferula arrigonii Bocchieri. Gazz. Chim. Ital. 1989, 11, 563. [Google Scholar]
- Serrilli, A.M.; Ramunno, A.; Amicucci, F.; Chicarella, V.; Santoni, S.; Ballero, M.; Serafini, M.; Bianco, A. Iridoidic pattern in endemic Sardinian plants: The case of Galium species. Nat. Prod. Res. 2008, 22, 618–622. [Google Scholar] [CrossRef]
- Harborne, J.B. Chemosystematics of the Leguminosae. Flavonoid and isoflavonoid patterns in the tribe Genisteae. Phytochemistry 1969, 8, 1449–1456. [Google Scholar] [CrossRef]
- Bernasconi, R.; Gill, S.; Steinegg, E. Versuch einer chemotaxonomisch-phylogenetischen Gliederung des Genus Genista. I. Quantitative Alkaloidverteilung in 26 Genista-Arten und Varietäten (8 Mitteilung über Leguminosenalkaloide). Pharm. Acta Helv. 1965, 40, 246–256. [Google Scholar]
- Pistelli, L.; Giachi, I.; Potenza, D.; Morelli, I. A new isoflavone from Genista corsica. J. Nat. Prod. 2000, 63, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Bertoli, A.; Giachi, I.; Manunta, A. Flavonoids from Genista ephedroides. J. Nat. Prod. 1998, 61, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Pistelli, L.; Bertoli, A.; Giachi, I.; Morelli, I.; Rubiolo, P.; Bicchi, C. Quinolizidine alkaloids from Genista ephedroides. Biochem. Syst. Ecol. 2001, 29, 137–141. [Google Scholar] [CrossRef]
- Giachi, I.; Manunta, A.; Morelli, I.; Pistelli, L. Flavonoids and isoflavonoids from Genista morisii. Biochem. Syst. Ecol. 2002, 30, 801–803. [Google Scholar] [CrossRef]
- Noccioli, C.; Meini, L.; Loi, M.C.; Potenza, D.; Pistelli, L. A new alpinumisoflavone derivative from Genista pichisermolliana. Phytochem. Lett. 2011, 4, 342–344. [Google Scholar] [CrossRef]
- Noccioli, C.; Luciardi, L.; Barsellini, S.; Favro, C.; Bertoli, A.; Bader, A.; Loi, M.C.; Pistelli, L. Flavonoids from two Italian genista species: Genista cilentina and Genista sulcitana. Chem. Nat. Compd. 2012, 48, 672–673. [Google Scholar] [CrossRef]
- Giamperi, L.; Fraternale, D.; Cara, P.; Ricci, D.; Epifano, F.; Curini, M. Analysis of essential oils from wild and domesticated plants of Glechoma sardoa Bég. J. Essent. Oil Res. 2008, 20, 38–40. [Google Scholar] [CrossRef]
- Satta, M.; Tuberoso, C.I.G.; Angioni, A.; Pirisi, F.M.; Cabras, P. Analysis of the essential oil of Helichrysum ifalicum G. Don ssp. microphyllum (Willd) Nym. J. Essent. Oil Res. 1999, 11, 711–715. [Google Scholar] [CrossRef]
- Bianchini, A.; Tomi, P.; Bernardini, A.F.; Morelli, I.; Flamini, G.; Cioni, P.L.; Usaï, M.; Marchetti, M. A comparative study of volatile constituents of two Helichrysum italicum (Roth) Guss. Don Fil subspecies growing in Corsica (France), Tuscany and Sardinia (Italy). Flavour Fragr. J. 2003, 18, 487–491. [Google Scholar] [CrossRef]
- Perrini, R.; Morone-Fortunato, I.; Lorusso, E.; Avato, P. Glands, essential oils and in vitro establishment of Helichrysum italicum (Roth) G. Don ssp. microphyllum (Willd.) Nyman. Ind. Crops Prod. 2009, 29, 395–403. [Google Scholar] [CrossRef]
- Melito, S.; Sias, A.; Petretto, G.L.; Chessa, M.; Pintore, G.; Porceddu, A. Genetic and metabolite diversity of Sardinian populations of Helichrysum italicum. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovanelli, S.; De Leo, M.; Cervelli, C.; Ruffoni, B.; Ciccarelli, D.; Pistelli, L. Essential Oil Composition and Volatile Profile of Seven Helichrysum Species Grown in Italy. Chem. Biodivers. 2018, 15. [Google Scholar] [CrossRef]
- Bianco, A.; Guiso, M.; Mazzei, R.A.; Piccioni, F.; Nicoletti, M.; Serafini, M.; Ballero, M. 6′-O-acetylantirrhinoside, a new iridoid glucoside from Linaria flava subsp. sardoa. Fitoterapia 1996, 67, 364–366. [Google Scholar]
- Fancello, F.; Zara, S.; Petretto, G.L.; Chessa, M.; Addis, R.; Rourke, J.P.; Pintore, G. Essential oils from three species of Mentha harvested in Sardinia: Chemical characterization and evaluation of their biological activity. Int. J. Food Prop. 2017, 20, 1751–1761. [Google Scholar] [CrossRef] [Green Version]
- Marengo, A.; Maxia, A.; Sanna, C.; Mandrone, M.; Bertea, C.M.; Bicchi, C.; Sgorbini, B.; Cagliero, C.; Rubiolo, P. Intra-specific variation in the little-known Mediterranean plant Ptilostemon casabonae (L.) Greuter analysed through phytochemical and biomolecular markers. Phytochemistry 2019, 161, 21–27. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Cioni, P.L.; Amadei, L.; Maccioni, S.; Flamini, G. Geographical patterns of in vivo spontaneously emitted volatile organic compounds in Salvia species. Microchem. J. 2017, 133, 13–21. [Google Scholar] [CrossRef]
- Rapposelli, E.; Melito, S.; Barmina, G.G.; Foddai, M.; Azara, E.; Scarpa, G.M. Relationship between soil and essential oil profiles in Salvia desoleana populations: Preliminary results. Nat. Prod. Commun. 2015, 10, 1615–1618. [Google Scholar] [CrossRef] [Green Version]
- Poli, F.; Bonsignore, L.; Loy, G.; Sacchetti, G.; Ballero, M. Comparison between the essential oils of Santolina insularis (Genn. ex Fiori) Arrigoni and Santolina corsica Jord. et Fourr. from the island of Sardinia (Italy). J. Ethnopharmacol. 1997, 56, 201–208. [Google Scholar] [CrossRef]
- Ferrari, B.; Tomi, F.; Richomme, P.; Casanova, J. Two new irregular acyclic sesquiterpenes aldehydes from Santolina corsica essential oil. Magn. Reson. Chem. 2005, 43, 73–74. [Google Scholar] [CrossRef]
- Ferrari, B.; Tomi, F.; Richomme, P.; Casanova, J. Terpenes and acetylene derivatives from the roots of Santolina corsica (Asteraceae). Biochem. Syst. Ecol. 2005, 33, 445–449. [Google Scholar] [CrossRef]
- Fattorusso, E.; Santelia, F.U.; Appendino, G.; Ballero, M.; Taglialatela-Scafati, O. Polyoxygenated Eudesmanes and trans-Chrysanthemanes from the Aerial Parts of Santolina insularis. J. Nat. Prod. 2004, 67, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ramunno, A.; Serrilli, A.M.; Piccioni, F.; Serafini, M.; Ballero, M. Taxonomical markers in two endemic plants of Sardinia: Verbascum conocarpum and Scrophularia trifoliata. Nat. Prod. Res. 2006, 20, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Bellino, A.; Venturella, P.; Luisa Marino, M.; Servettaz, O.; Venturella, G. Coumarins from Seseli bocconi. Phytochemistry 1986, 25, 1195–1199. [Google Scholar] [CrossRef]
- Serrilli, A.M.; Ramunno, A.; Piccioni, F.; Serafini, M.; Ballero, M. Flavonoids and iridoids from Stachys corsica. Nat. Prod. Res. 2005, 19, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Peruzzi, L.; Menichini, F. Phytochemical and biological studies of Stachys species in relation to chemotaxonomy: A review. Phytochemistry 2014, 102, 7–39. [Google Scholar] [CrossRef] [PubMed]
- Serrilli, A.M.; Ramunno, A.; Piccioni, F.; Serafini, M.; Ballero, M.; Bianco, A. Monoterpenoids from Stachys glutinosa L. Nat. Prod. Res. 2006, 20, 648–652. [Google Scholar] [CrossRef]
- Mariotti, J.P.; Costa, J.; Bianchini, A.; Bernardini, A.F.; Casanova, J. Composition and Variability of the Essential Oil of Stachys glutinosa L. from Corsica (France). Flavour Fragr. J. 1997, 12, 205–209. [Google Scholar] [CrossRef]
- Corticchiato, M.; Bernardini, A.; Costa, J.; Bayet, C.; Saunois, A.; Voirin, B. Free flavonoid aglycones from Thymus herba barona and its monoterpenoid chemotypes. Phytochemistry 1995, 40, 115–120. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Sanna, C.; Rubiolo, P.; Basilico, N.; Colombo, E.; Scaltrito, M.; Ndiath, O.; Maccarone, L.; Taramelli, D.; Bicchi, C.; et al. Anti-plasmodial and insecticidal activities of the essential oils of aromatic plants growing in the Mediterranean area. Malar. J. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- Crippa, S.; Danieli, B.; Lesma, G.; Palmisano, G.; Ferrari, G.; Vecchietti, V. Indole alkaloids from Vinca sardoa, a new species of Vinca. Heterocycles 1990, 31, 1663–1667. [Google Scholar]
- Nicoletti, M.; Serafini, M.; Federici, E.; Galeffi, C.; Poli, F. Indole alkaloids from aerial parts of Vinca sardoa. Phytochemistry 1998, 47, 149–151. [Google Scholar] [CrossRef]
- Bianco, A.; Guiso, M.; Nicoletti, M.; Foddai, S.; Piccin, A.; Serafini, M.; Ballero, M.; Poli, F. A comparative chemotaxonomic study on Vinca sardoa Stearn and Vinca difformis Pourret. Nat. Prod. Res. 2005, 19, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Ballero, M.; Serafini, M.; Romagnoli, C.; Bruni, A.; Poli, F. Laticifer tissue distribution and alkaloid location in Vinca sardoa (Stearn) Pign. (Apocynaceae), an endemic plant of Sardinia (Italy). Phyt. Ann. Rei Bot. 1999, 39, 265–275. [Google Scholar]
- Danieli, B.; Lesma, G.; Palmisano, G.; Ferrari, G.; Vecchietti, V.; Picci, V.; Atzei, A. Indole alkaloids from Vinca sardoa, a new species of Vinca. Planta Med. 1980, 39, 207. [Google Scholar]
- Verpoorte, R. Exploration of nature’s chemodiversity: The role of secondary metabolites as leads in drug development. Drug Discov. Today 1998, 3, 232–238. [Google Scholar] [CrossRef]
- Schippmann, U.; Leaman, D.; Cunningham, A.B. A Comparison of Cultivation and Wild Collection of Medicinal and Aromatic Plants under Sustainability Aspects. Med. Aromat. Plants 2007, 75–95. [Google Scholar] [CrossRef]
- Cogoni, D.; Sulis, E.; Bacchetta, G.; Fenu, G. The unpredictable fate of the single population of a threatened narrow endemic Mediterranean plant. Biodivers. Conserv. 2019, 28, 1799–1813. [Google Scholar] [CrossRef]
- Fenu, G.; Cogoni, D.; Ulian, T.; Bacchetta, G. The impact of human trampling on a threatened coastal Mediterranean plant: The case of Anchusa littorea Moris (Boraginaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 104–110. [Google Scholar] [CrossRef]
- Orsenigo, S.; Montagnani, C.; Fenu, G.; Gargano, D.; Peruzzi, L.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Bovio, M.; et al. Red Listing plants under full national responsibility: Extinction risk and threats in the vascular flora endemic to Italy. Biol. Conserv. 2018, 224, 213–222. [Google Scholar] [CrossRef]
- Porceddu, M.; Sanna, M.; Serra, S.; Manconi, M.; Bacchetta, G. Seed germination requirements of Hypericum scruglii, an endangered medicinal plant species of Sardinia (Italy). Botany 2020, cjb-2020-0039. [Google Scholar] [CrossRef]
- Fenu, G.; Bacchetta, G.; Charalambos, S.C.; Fournaraki, C.; Giusso del Galdo, G.P.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; Pinna, M.S.; et al. An early evaluation of translocation actions for endangered plant species on Mediterranean islands. Plant Divers. 2019, 41, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Family | Taxon | Distribution Range |
|---|---|---|
| Amaryllidaceae | Pancratium illyricum L. | SA-CO-AT |
| Apiaceae | Ferula arrigonii Bocchieri | SA-CO |
| Seseli praecox (Gamisans) Gamisans | SA-CO | |
| Apocynaceae | Vinca difformis Pourr. subsp. sardoa Stearn | SA |
| Araceae | Arum pictum L.f. subsp. pictum | SA-CO |
| Aristolochiaceae | Aristolochia tyrrhena E.Nardi & Arrigoni | SA-CO-AT |
| Asteraceae | Artemisia caerulescens L. subsp. densiflora (Viv.) Kerguélen & Lambinon | SA-CO |
| Centaurea horrida Badarò | SA | |
| Helichrysum italicum (Roth) G.Don subsp. tyrrhenicum (Bacch., Brullo & Giusso) Herrando, J.M.Blanco, L.Sáez Galbany | SA-CO | |
| Helichrysum saxatile Moris subsp. saxatile | SA | |
| Plagius flosculosus (L.) Alavi & Heywood | SA-CO-AT | |
| Ptilostemon casabonae (L.) Greuter | SA-CO-HI | |
| Santolina corsica Jord. & Fourr. | SA-CO | |
| Santolina insularis (Gennari ex Fiori) Arrigoni | SA | |
| Tanacetum audibertii (Req.) DC. | SA-CO | |
| Boraginaceae | Borago morisiana Bigazzi & Ricceri | SA |
| Borago pygmaea (DC.) Chater & Greuter | SA-CO-AT | |
| Euphorbiaceae | Euphorbia hyberna L. subsp. insularis (Boiss.) Briq. | SA-CO-LI-TO |
| Euphorbia pithyusa L. subsp. cupanii (Guss. ex Bertol.) Radcl.-Sm. | SA-CO-SI | |
| Euphorbia semiperfoliata Viv. | SA-CO | |
| Fabaceae | Astragalus verrucosus Moris | SA |
| Bituminaria morisiana (Pignatti & Metlesics) Greuter | SA-TN | |
| Genista arbusensis Vals. | SA | |
| Genista bocchierii Bacch., Brullo & Feoli Chiapella | SA | |
| Genista cadasonensis Vals. | SA | |
| Genista corsica (Loisel.) DC. | SA-CO | |
| Genista ephedroides DC. | SA-CO | |
| Genista morisii Colla | SA | |
| Genista pichisermolliana Vals. | SA | |
| Genista sulcitana Vals. | SA | |
| Hypericaceae | Hypericum hircinum L. subsp. hircinum | SA-CO-AT |
| Hypericum scruglii Bacch., Brullo & Salmeri | SA | |
| Lamiaceae | Glechoma sardoa (Bég.) Bég. | SA |
| Mentha requienii Benth. subsp. requienii | SA-CO | |
| Salvia desoleana Atzei & V.Picci | SA | |
| Stachys corsica Pers. | SA-CO | |
| Stachys glutinosa L. | SA-CO-AT | |
| Thymus herba-barona Loisel. subsp. herba-barona | SA-CO | |
| Orobanchaceae | Euphrasia nana (Rouy) Prain | SA-CO |
| Plantaginaceae | Cymbalaria muelleri (Moris) A.Chev. | SA |
| Linaria flava (Poir.) Desf. subsp. sardoa (Sommier) A.Terracc. | SA-CO | |
| Plumbaginaceae | Limonium contortirameum (Mabille) Erben | SA-CO |
| Limonium morisianum Arrigoni | SA | |
| Polygalaceae | Polygala sardoa Chodat | SA |
| Ranunculaceae | Staphisagria requienii (DC.) Spach subsp. picta (Willd.) Peruzzi | SA-CO-BL-HI |
| Rubiaceae | Galium corsicum Spreng. | SA-CO |
| Galium glaucophyllum Em.Schmid | SA | |
| Galium schmidii Arrigoni | SA | |
| Scrophulariaceae | Scrophularia trifoliata L. | SA-CO-AT |
| Verbascum conocarpum Moris subsp. conocarpum | SA-CO-AT | |
| Urticaceae | Urtica atrovirens Req. ex Loisel. | SA-CO-AT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, C.; Maxia, A.; Fenu, G.; Loi, M.C. So Uncommon and so Singular, but Underexplored: An Updated Overview on Ethnobotanical Uses, Biological Properties and Phytoconstituents of Sardinian Endemic Plants. Plants 2020, 9, 958. https://doi.org/10.3390/plants9080958
Sanna C, Maxia A, Fenu G, Loi MC. So Uncommon and so Singular, but Underexplored: An Updated Overview on Ethnobotanical Uses, Biological Properties and Phytoconstituents of Sardinian Endemic Plants. Plants. 2020; 9(8):958. https://doi.org/10.3390/plants9080958
Chicago/Turabian StyleSanna, Cinzia, Andrea Maxia, Giuseppe Fenu, and Maria Cecilia Loi. 2020. "So Uncommon and so Singular, but Underexplored: An Updated Overview on Ethnobotanical Uses, Biological Properties and Phytoconstituents of Sardinian Endemic Plants" Plants 9, no. 8: 958. https://doi.org/10.3390/plants9080958