Pharmacological and Advanced Cell Respiration Effects, Enhanced by Toxic Human-Bile Nano-Pharmaceuticals of Probucol Cell-Targeting Formulations
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Drug Preparations
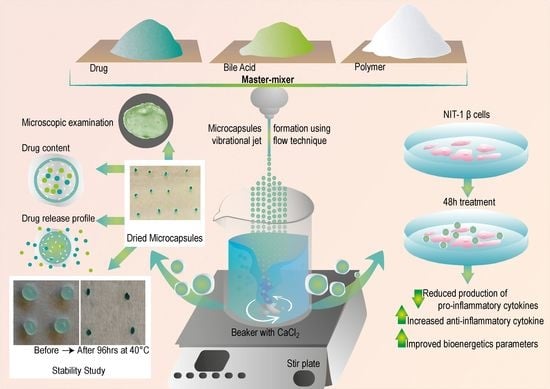
2.3. Microcapsules Preparation
2.4. Characterization of Loaded Microcapsules
2.4.1. Morphological Analysis and Surface Characterization of Microcapsules
2.4.2. Drug Content, Production Yield, and Microencapsulation Efficiency
2.4.3. Electrokinetic Stability, Size Analysis, Surface Tension and Conductivity
2.4.4. Swelling and Mechanical Resistance Studies
2.4.5. Buoyancy Test
2.4.6. Drug Release Studies—In Vitro Dissolution Test
2.4.7. Physical Stability
2.4.8. NIT-1 Pancreatic β Cells and Biological Analysis
2.4.9. Statistical Analysis
3. Results and Discussion
3.1. Microscopic Examination and Surface Analysis
3.2. Drug Content, Microencapsulation Efficiency, Production Yield, Zeta Potential, Size Analysis, Surface Tension, and Conductivity
3.3. Swelling Index
3.4. Mechanical Strength, Buoyancy Test and Drug Release Studies
3.5. Stability Studies
3.6. Biological Activity of PB-Loaded Microcapsules
3.6.1. Pancreatic β-Cell Cytokine Measurement
3.6.2. Seahorse Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, H.; Aubert, R.E.; Herman, W.H. Global burden of diabetes, 1995–2025: Prevalence, numerical estimates, and projections. Diabetes Care 1998, 21, 1414–1431. [Google Scholar] [CrossRef]
- Alberti, K. The World Health Organisation and Diabetes; Springer: Berlin, Germany, 1980. [Google Scholar]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H., Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Organization, W.H. World Health Organization Diabetes Fact Sheet; WHO: Geneva, Switzerland, 2011. [Google Scholar]
- Johansen, J.S.; Harris, A.K.; Rychly, D.J.; Ergul, A. Oxidative stress and the use of antioxidants in diabetes: Linking basic science to clinical practice. Cardiovasc. Diabetol. 2005, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, P.A.; Zgibor, J.C.; Dasanayake, A.P. Diabetes: A growing epidemic of all ages. J. Am. Dent. Assoc. 2003, 134, 11S–15S. [Google Scholar] [CrossRef] [PubMed]
- Maritim, A.C.; Sanders, A.; Watkins III, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Oberley, L.W. Free radicals and diabetes. Free Radic. Biol. Med. 1988, 5, 113–124. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef] [Green Version]
- Schmid, K.L.; Schmid, L.M. Knowledge of the ocular effects of diabetes among the general population of Australia and the members of Diabetes Australia. Clin. Exp. Optom. 2003, 86, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Mooranian, A.; Negrulj, R.; Mikov, M.; Golocorbin-Kon, S.; Arfuso, F.; Al-Salami, H. Novel chenodeoxycholic acid-sodium alginate matrix in the microencapsulation of the potential antidiabetic drug, probucol. An in vitro study. J. Microencapsul 2015, 32, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Angus, R.; Madsen, B.; Britt, D.; Vernon, B.; Nguyen, K.T. Islet Encapsulation: Strategies to Enhance Islet Cell Functions. Tissue Eng. 2007, 13, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Matsuzawa, Y. Where are we with probucol: A new life for an old drug? Atherosclerosis 2009, 207, 16–23. [Google Scholar] [CrossRef]
- Heel, R.; Brogden, R.; Speight, T.; Avery, G. Probucol: A review of its pharmacological properties and therapeutic use in patients with hypercholesterolaemia. Drugs 1978, 15, 409–428. [Google Scholar] [CrossRef]
- Crim, W.S.; Wu, R.; Carter, J.D.; Cole, B.K.; Trace, A.P.; Mirmira, R.G.; Kunsch, C.; Nadler, J.L.; Nunemaker, C.S. AGI-1067, a novel antioxidant and anti-inflammatory agent, enhances insulin release and protects mouse islets. Mol. Cell. Endocrinol. 2010, 323, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Davignon, J. Probucol. In Principles and Treatment of Lipoprotein Disorders; Springer: Berlin, Germany, 1994; pp. 429–469. [Google Scholar]
- Russell, J.C.; Graham, S.E.; Amy, R.M.; Dolphin, P.J. Cardioprotective effect of probucol in the atherosclerosis-prone JCR:LA-cp rat. Eur. J. Pharmacol. 1998, 350, 203–210. [Google Scholar] [CrossRef]
- Kaneto, H.; Katakami, N.; Kawamori, D.; Miyatsuka, T.; Sakamoto, K.Y.; Matsuoka, T.-A.; Matsuhisa, M.; Yamasaki, Y. Involvement of oxidative stress in the pathogenesis of diabetes. Antioxid. Redox Signal. 2007, 9, 355–366. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and glycemic control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Gorogawa, S.-I.; Kajimoto, Y.; Umayahara, Y.; Kaneto, H.; Watada, H.; Kuroda, A.; Kawamori, D.; Yasuda, T.; Matsuhisa, M.; Yamasaki, Y. Probucol preserves pancreatic β-cell function through reduction of oxidative stress in type 2 diabetes. Diabetes Res. Clin. Pract. 2002, 57, 1–10. [Google Scholar] [CrossRef]
- Takatori, A.; Ohta, E.; Inenaga, T.; Horiuchi, K.; Ishii, Y.; Itagaki, S.-I.; Kyuwa, S.; Yoshikawa, Y. Protective effects of probucol treatment on pancreatic β-cell function of SZ-induced diabetic APA hamsters. Exp. Anim. 2003, 52, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Zimetbaum, P.; Eder, H.; Frishman, W. Probucol: Pharmacology and clinical application. J. Clin. Pharmacol. 1990, 30, 3–9. [Google Scholar] [CrossRef]
- Heeg, J.F.; Hiser, M.F.; Satonin, D.K.; Rose, J.Q. Pharmacokinetics of probucol in male rats. J. Pharm. Sci. 1984, 73, 1758–1763. [Google Scholar] [CrossRef]
- Matsushita, M.; Yoshino, G.; Iwai, M.; Matsuba, K.; Morita, M.; Iwatani, I.; Yoshida, M.; Kazumi, T.; Baha, S. Protective effect of probucol on alloxan diabetes in rats. Diabetes Res. Clin. Pract. 1989, 7, 313–316. [Google Scholar] [CrossRef]
- Liu, J.H.; Liu, D.F.; Wang, N.N.; Lin, H.L.; Mei, X. Possible role for the thioredoxin system in the protective effects of probucol in the pancreatic islets of diabetic rats. Clin. Exp. Pharmacol. Physiol. 2011, 38, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. The effect of a tertiary bile acid, taurocholic acid, on the morphology and physical characteristics of microencapsulated probucol: Potential applications in diabetes: A characterization study. Drug Deliv. Transl. Res. 2015, 5, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Multicompartmental, multilayered probucol microcapsules for diabetes mellitus: Formulation characterization and effects on production of insulin and inflammation in a pancreatic β-cell line. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1642–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Watts, G.F.; Arfuso, F.; Al-Salami, H. An optimized probucol microencapsulated formulation integrating a secondary bile acid (deoxycholic acid) as a permeation enhancer. Drug Des. Dev. Ther. 2014, 8, 1673. [Google Scholar]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. A comprehensive study of novel microcapsules incorporating gliclazide and a permeation enhancing bile acid: Hypoglycemic effect in an animal model of Type-1 diabetes. Drug Deliv. 2016, 23, 2869–2880. [Google Scholar] [CrossRef] [PubMed]
- Mikov, M.; Al-Salami, H.; Golocorbin-Kon, S.; Skrbic, R.; Raskovic, A.; Fawcett, J.P. The influence of 3alpha,7alpha-dihydroxy-12-keto-5beta-cholanate on gliclazide pharmacokinetics and glucose levels in a rat model of diabetes. Eur. J. Drug Metab. Pharmacokinet. 2008, 33, 137–142. [Google Scholar] [CrossRef]
- Al-Salami, H.; Butt, G.; Tucker, I.; Golocorbin-Kon, S.; Mikov, M. Probiotics decreased the bioavailability of the bile acid analog, monoketocholic acid, when coadministered with gliclazide, in healthy but not diabetic rats. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 99–108. [Google Scholar] [CrossRef]
- Al-Salami, H.; Butt, G.; Tucker, I.; Fawcett, P.J.; Golo-Corbin-Kon, S.; Mikov, I.; Mikov, M. Gliclazide reduces MKC intestinal transport in healthy but not diabetic rats. Eur. J. Drug Metab. Pharmacokinet. 2009, 34, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Al-Salami, H.; Butt, G.; Tucker, I.; Mikov, M. Influence of the semisynthetic bile acid MKC on the ileal permeation of gliclazide in vitro in healthy and diabetic rats treated with probiotics. Methods Find. Exp. Clin. Pharmacol. 2008, 30, 107–114. [Google Scholar] [CrossRef]
- Al-Salami, H.; Butt, G.; Tucker, I.; Mikov, M. Probiotic treatment proceeded by a single dose of bile acid and gliclazide exert the most hypoglycemic effect in Type 1 diabetic rats. Med. Hypothesis Res. 2008, 4, 93–101. [Google Scholar]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Fakhoury, M.; Arfuso, F.; Jones, F.; Al-Salami, H. Advanced bile acid-based multi-compartmental microencapsulated pancreatic β-cells integrating a polyelectrolyte-bile acid formulation, for diabetes treatment. Artif. Cells Nanomed. Biotechnol. 2016, 44, 588–595. [Google Scholar] [CrossRef]
- Moretti, A.; Li, Q.; Chmielowski, R.; Joseph, L.B.; Moghe, P.V.; Uhrich, K.E. Nanotherapeutics containing lithocholic acid-based amphiphilic scorpion-like macromolecules reduce in vitro inflammation in macrophages: Implications for atherosclerosis. Nanomaterials 2018, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagle, S.R.; Walker, D.; Kovacevic, B.; Gedawy, A.; Mikov, M.; Golocorbin-Kon, S.; Mooranian, A.; Al-Salami, H. Micro-nano formulation of bile-gut delivery: Rheological, stability and cell survival, basal and maximum respiration studies. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Staudinger, J.L.; Goodwin, B.; Jones, S.A.; Hawkins-Brown, D.; MacKenzie, K.I.; LaTour, A.; Liu, Y.; Klaassen, C.D.; Brown, K.K.; Reinhard, J.; et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. USA 2001, 98, 3369–3374. [Google Scholar] [CrossRef] [Green Version]
- Adachi, R.; Honma, Y.; Masuno, H.; Kawana, K.; Shimomura, I.; Yamada, S.; Makishima, M. Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. J. Lipid Res. 2005, 46, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Chiang, J.Y.L. Nuclear receptors in bile acid metabolism. Drug Metab. Rev. 2013, 45, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Kolluru, L.P.; Gala, R.P. Design of Experiments: A Valuable “Quality by Design” Tool in Formulation Development. Nanopart. Vaccine Deliv. Syst. 2015, 61. [Google Scholar] [CrossRef]
- Wei, X.; Liao, J.; Davoudi, Z.; Zheng, H.; Chen, J.; Li, D.; Xiong, X.; Yin, Y.; Yu, X.; Xiong, J.; et al. Folate Receptor-Targeted and GSH-Responsive Carboxymethyl Chitosan Nanoparticles Containing Covalently Entrapped 6-Mercaptopurine for Enhanced Intracellular Drug Delivery in Leukemia. Mar. Drugs 2018, 16, 439. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Yin, L.; Zhang, X.; Zhang, H.; Hu, R.; Yin, Y.; Qiu, T.; Xiong, X.; Wang, Q. Redox Sensitive Shell and Core Crosslinked Hyaluronic Acid Nanocarriers for Tumor-Targeted Drug Delivery. J. Biomed. Nanotechnol. 2016, 12, 1641–1653. [Google Scholar] [CrossRef]
- Poovaiah, N.; Davoudi, Z.; Peng, H.; Schlichtmann, B.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Treatment of neurodegenerative disorders through the blood-brain barrier using nanocarriers. Nanoscale 2018, 10, 16962–16983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoudi, Z.; Peroutka-Bigus, N.; Bellaire, B.; Wannemuehler, M.; Barrett, T.A.; Narasimhan, B.; Wang, Q. Intestinal organoids containing poly(lactic-co-glycolic acid) nanoparticles for the treatment of inflammatory bowel diseases. J. Biomed. Mater. Res. A 2018, 106, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Larsson, M.; Huang, W.-T.; Chiou, S.-H.; Nicholls, S.J.; Chao, J.-I.; Liu, D.-M. The use of polymer-based nanoparticles and nanostructured materials in treatment and diagnosis of cardiovascular diseases: Recent advances and emerging designs. Prog. Polym. Sci. 2016, 57, 153–178. [Google Scholar] [CrossRef]
- Whelehan, M.; Marison, I.W. Microencapsulation using vibrating technology. J. Microencapsul 2011, 28, 669–688. [Google Scholar] [CrossRef]
- Mathavan, S.; Chen-Tan, N.; Arfuso, F.; Al-Salami, H. The role of the bile acid chenodeoxycholic acid in the targeted oral delivery of the anti-diabetic drug gliclazide, and its applications in type 1 diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1508–1519. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Yin, B.-C.; Zhang, W.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Composite microparticle drug delivery systems based on chitosan, alginate and pectin with improved pH-sensitive drug release property. Colloids Surf. B Biointerfaces 2009, 68, 245–249. [Google Scholar] [CrossRef]
- Storz, H.; Müller, K.J.; Ehrhart, F.; Gómez, I.; Shirley, S.G.; Gessner, P.; Zimmermann, G.; Weyand, E.; Sukhorukov, V.L.; Forst, T. Physicochemical features of ultra-high viscosity alginates. Carbohyd Res. 2009, 344, 985–995. [Google Scholar] [CrossRef]
- Lee, H.Y.; Chan, L.W.; Dolzhenko, A.V.; Heng, P.W.S. Influence of viscosity and uronic acid composition of alginates on the properties of alginate films and microspheres produced by emulsification. J. Microencapsul 2006, 23, 912–927. [Google Scholar] [CrossRef]
- Choi, Y.S.; Hong, S.R.; Lee, Y.M.; Song, K.W.; Park, M.H.; Nam, Y.S. Study on gelatin-containing artificial skin: I. Preparation and characteristics of novel gelatin-alginate sponge. Biomaterials 1999, 20, 409–417. [Google Scholar] [CrossRef]
- Al-Salami, H.; Mamo, J.; Mooranian, A.; Negrulj, R.; Lam, V.; Elahy, M.; Takechi, R. Long-term supplementation of microencapsulated ursodeoxycholic acid prevents hypertension in a mouse model of insulin resistance. Exp. Clin. Endocrinol. Diabetes 2017, 125, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The incorporation of water-soluble gel matrix into bile acid-based microcapsules for the delivery of viable β-cells of the pancreas, in diabetes treatment: Biocompatibility and functionality studies. Drug Deliv. Transl. Res. 2016, 6, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Al-Sallami, H.S.; Fang, Z.; Mukkur, T.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F. Microencapsulation as a novel delivery method for the potential antidiabetic drug, Probucol. Drug Des. Dev. Ther. 2014, 8, 1221. [Google Scholar]
- Mooranian, A.; Negrulj, R.; Al-Sallami, H.S.; Fang, Z.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Arfuso, F.; Al-Salami, H. Release and swelling studies of an innovative antidiabetic-bile acid microencapsulated formulation, as a novel targeted therapy for diabetes treatment. J. Microencapsul 2015, 32, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Negrulj, R.; Mooranian, A.; Chen-Tan, N.; Al-Sallami, H.S.; Mikov, M.; Golocorbin-Kon, S.; Fakhoury, M.; Watts, G.F.; Arfuso, F.; Al-Salami, H. Swelling, mechanical strength, and release properties of probucol microcapsules with and without a bile acid, and their potential oral delivery in diabetes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1290–1297. [Google Scholar] [CrossRef]
- Nourooz-Zadeh, J.; Gopaul, N.K.; Forster, L.A.; Ferns, G.A.; Änggård, E.E. Measurement of plasma probucol levels by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1994, 654, 55–60. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Takechi, R.; Mamo, J.; Al-Sallami, H.; Al-Salami, H. The biological effects of the hypolipidaemic drug probucol microcapsules fed daily for 4 weeks, to an insulin-resistant mouse model: Potential hypoglycaemic and anti-inflammatory effects. Drug Deliv. Transl. Res. 2018, 8, 543–551. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Mathavan, S.; Martinez, J.; Sciarretta, J.; Chen-Tan, N.; Mukkur, T.; Mikov, M.; Lalic-Popovic, M.; Stojancevic, M. An advanced microencapsulated system: A platform for optimized oral delivery of antidiabetic drug-bile acid formulations. Pharm. Dev. Technol. 2015, 20, 702–709. [Google Scholar] [CrossRef]
- Barakat, N.S.; Shazly, G.A.; Almedany, A.H. Influence of polymer blends on the characterization of gliclazide–encapsulated into poly (Æ-caprolactone) microparticles. Drug Dev. Ind. Pharm. 2013, 39, 352–362. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Arfuso, F.; Al-Salami, H. Characterization of a novel bile acid-based delivery platform for microencapsulated pancreatic β-cells. Artif. Cells Nanomed. Biotechnol. 2016, 44, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, K.; Gaskins, H.R.; Leiter, E.H. NIT-1, a pancreatic β-cell line established from a transgenic NOD/Lt mouse. Diabetes 1991, 40, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Kulkarni, G.T. Development of novel gastroretentive floating particulate drug delivery system of gliclazide. Curr. Drug Deliv. 2012, 9, 437–451. [Google Scholar] [CrossRef]
- Mooranian, A.; Negrulj, R.; Al-Salami, H. The influence of stabilized deconjugated ursodeoxycholic acid on polymer-hydrogel system of transplantable NIT-1 cells. Pharm. Res. 2016, 33, 1182–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honary, S.; Zahir, F. Effect of zeta potential on the properties of nano-drug delivery systems-a review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Azarbayjani, A.F.; Jouyban, A.; Chan, S.Y. Impact of surface tension in pharmaceutical sciences. J. Pharm. Pharm. Sci. 2009, 12, 218–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.G.; Li, X.X.; Lv, G.J.; Xie, W.Y.; Zhu, J.; Luxbacher, T.; Ma, R.; Ma, X.J. Effect of surface wettability and charge on protein adsorption onto implantable alginate-chitosan-alginate microcapsule surfaces. J. Biomed. Mater. Res. A 2010, 92, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Roda, A. Physicochemical properties of bile acids and their relationship to biological properties: An overview of the problem. J. Lipid Res. 1984, 25, 1477–1489. [Google Scholar] [PubMed]
- Al-Kassas, R.S.; Al-Gohary, O.M.; Al-Faadhel, M.M. Controlling of systemic absorption of gliclazide through incorporation into alginate beads. Int. J. Pharm. 2007, 341, 230–237. [Google Scholar] [CrossRef]
- Mathavan, S.; Mikov, M.; Golocorbin-Kon, S.; Al-Salami, H. Diabetes development increased concentrations of the conjugated bile acid, taurocholic acid in serum, while treatment with microencapsulated-taurocholic acid exerted no hypoglycaemic effects. Eur. J. Pharm. Sci. 2017, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Al-Salami, H.; Butt, G.; Tucker, I.; Skrbic, R.; Golocorbin-Kon, S.; Mikov, M. Probiotic Pre-treatment Reduces Gliclazide Permeation (ex vivo) in Healthy Rats but Increases It in Diabetic Rats to the Level Seen in Untreated Healthy Rats. Arch. Drug Inf. 2008, 1, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luner, P.E. Wetting properties of bile salt solutions and dissolution media. J. Pharm. Sci. 2000, 89, 382–395. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, H.; Mikov, M.; Tucker, I.G. Physicochemical and biological characterization of monoketocholic acid, a novel permeability enhancer. Mol. Pharm. 2009, 6, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Inflammatory cytokines and the risk to develop type 2 diabetes: Results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Karunakaran, U.; Park, K.-G. A systematic review of oxidative stress and safety of antioxidants in diabetes: Focus on islets and their defense. Diabetes Metab. J. 2013, 37, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.B.J.; Lajczak, N.K.; Kelly, O.B.; O’Dwyer, A.M.; Giddam, A.K.; Gabhann, J.N.; Franco, P.; Tambuwala, M.M.; Jefferies, C.A.; Keely, S.; et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G550–G558. [Google Scholar] [CrossRef] [Green Version]
- Koh, S.J.; Kim, J.M.; Kim, I.K.; Ko, S.H.; Kim, J.S. Anti-inflammatory mechanism of metformin and its effects in intestinal inflammation and colitis-associated colon cancer. J. Gastroenterol. Hepatol. 2014, 29, 502–510. [Google Scholar] [CrossRef]
- Mooranian, A.; Wagle, S.R.; Kovacevic, B.; Takechi, R.; Mamo, J.; Lam, V.; Watts, G.F.; Mikov, M.; Golocorbin-Kon, S.; Stojanovic, G.; et al. Bile acid bio-nanoencapsulation improved drug targeted-delivery and pharmacological effects via cellular flux: 6-months diabetes preclinical study. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Negrulj, R.; Mooranian, A.; Al-Salami, H. Potentials and limitations of bile acids in type 2 diabetes mellitus: Applications of microencapsulation as a novel oral delivery system. J. Endocrinol. Diabetes Mellit. 2013, 1, 49–59. [Google Scholar]
- Stojančević, M.; Pavlović, N.; Goločorbin-Kon, S.; Mikov, M. Application of bile acids in drug formulation and delivery. Front. Life Sci. 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikstrom, J.D.; Sereda, S.B.; Stiles, L.; Elorza, A.; Allister, E.M.; Neilson, A.; Ferrick, D.A.; Wheeler, M.B.; Shirihai, O.S. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PLoS ONE 2012, 7, e33023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mooranian, A.; Tackechi, R.; Jamieson, E.; Morahan, G.; Al-Salami, H. Innovative Microcapsules for Pancreatic beta-Cells Harvested from Mature Double-Transgenic Mice: Cell Imaging, Viability, Induced Glucose-Stimulated Insulin Measurements and Proinflammatory Cytokines Analysis. Pharm. Res. 2017, 34, 1217–1223. [Google Scholar] [CrossRef] [PubMed]





| Temperature = 25 °C (A) | ||||
| Formula Code | pH 1.5 | pH 3 | pH 6 | pH 7.8 |
| F1 | 0.92 ± 0.005 | 1.873 ± 0.0625 * | 3.286 ± 0.148 * | 3.90 ± 0.11 * |
| F2 | 0.89 ± 0.005 | 1.383 ± 0.343 * | 2.633 ± 0.104 * | 3.08 ± 0.05 * |
| Temperature = 37°C (B) | ||||
| F1 | 0.99 ± 0.005 | 2.345 ± 0.005 * | 3.83 ± 0.056 * | 4.89 ± 0.095 * |
| F2 | 0.933 ± 0.057 | 2.12 ± 0.081 * | 2.986 ± 0.349 * | 3.87 ± 0.161 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagle, S.R.; Kovacevic, B.; Walker, D.; Ionescu, C.M.; Jones, M.; Stojanovic, G.; Kojic, S.; Mooranian, A.; Al-Salami, H. Pharmacological and Advanced Cell Respiration Effects, Enhanced by Toxic Human-Bile Nano-Pharmaceuticals of Probucol Cell-Targeting Formulations. Pharmaceutics 2020, 12, 708. https://doi.org/10.3390/pharmaceutics12080708
Wagle SR, Kovacevic B, Walker D, Ionescu CM, Jones M, Stojanovic G, Kojic S, Mooranian A, Al-Salami H. Pharmacological and Advanced Cell Respiration Effects, Enhanced by Toxic Human-Bile Nano-Pharmaceuticals of Probucol Cell-Targeting Formulations. Pharmaceutics. 2020; 12(8):708. https://doi.org/10.3390/pharmaceutics12080708
Chicago/Turabian StyleWagle, Susbin Raj, Bozica Kovacevic, Daniel Walker, Corina Mihaela Ionescu, Melissa Jones, Goran Stojanovic, Sanja Kojic, Armin Mooranian, and Hani Al-Salami. 2020. "Pharmacological and Advanced Cell Respiration Effects, Enhanced by Toxic Human-Bile Nano-Pharmaceuticals of Probucol Cell-Targeting Formulations" Pharmaceutics 12, no. 8: 708. https://doi.org/10.3390/pharmaceutics12080708