Abstract
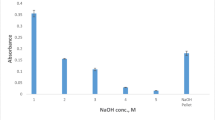
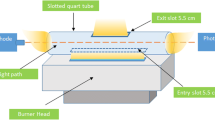
In this study, a vortex-assisted switchable solvent microextraction (VA-SSME) method was developed for the determination of iron by slotted quartz tube flame atomic absorption spectrometry (SQT-FAAS). A ligand synthesized from the reaction of ortho-phenylenediamine and 5-bromosalicylaldehyde was used to form a coordinate complex of iron. All experimental variables such as switchable solvent amount, sodium hydroxide concentration, sodium hydroxide amount, and diluent amount were optimized to increase extraction efficiency for the iron complex. Optimum conditions were applied to aqueous standard solutions in the range of 20–750 ng/mL, and the percent relative standard deviation (%RSD) was less than 2.0%. The limit of detection (LOD) and limit of quantification (LOQ) were determined as 4.8 and 16.2 ng/mL, respectively. The optimized method recorded approximately 53 times enhancement according to the conventional FAAS system. The proposed method was applied to mineral spring water samples, and satisfactory percent recovery results (100–105%) were obtained for iron, indicating good applicability in addition to high accuracy.



Similar content being viewed by others
References
Abdel-Azeem, S. M., Bader, N. R., Kuss, H. M., & El-Shahat, M. F. (2013). Determination of total iron in food samples after flow injection preconcentration on polyurethane foam functionalized with N,N-bis(salicylidene)- 1,3-propanediamine. Food Chemistry, 138(2–3), 1641–1647. https://doi.org/10.1016/j.foodchem.2012.11.054.
Abduljabbar, T. N., Sharp, B. L., Reid, H. J., Barzegar-Befroeid, N., Peto, T., & Lengyel, I. (2019). Determination of Zn, Cu and Fe in human patients’ serum using micro-sampling ICP-MS and sample dilution. Talanta, 204, 663–669. https://doi.org/10.1016/J.TALANTA.2019.05.098.
Atsever, N., Borahan, T., Gülhan Bakırdere, E., & Bakırdere, S. (2020). Determination of iron in hair samples by slotted quartz tube-flame atomic absorption spectrometry after switchable solvent liquid phase extraction. Journal of Pharmaceutical and Biomedical Analysis, 186, 113274. https://doi.org/10.1016/j.jpba.2020.113274.
Bakırdere, E. G., Akarçay, N. A., Zaman, B. T., & Bakırdere, S. (n.d.). A novel and efficient analytical method for trace cobalt determination by dispersive liquid-liquid microextraction basedsimultaneous complexation and extraction prior to slotted quartz tube flame atomic absorption spectrometry in chamomile samples.
Borzoei, M., Zanjanchi, M. A., Sadeghi-aliabadi, H., & Saghaie, L. (2018). Optimization of a methodology for determination of iron concentration in aqueous samples using a newly synthesized chelating agent in dispersive liquid-liquid microextraction. Food Chemistry, 264, 9–15. https://doi.org/10.1016/J.FOODCHEM.2018.04.135.
de Oliveira Souza, M., Ribeiro, M. A., Carneiro, M. T. W. D., Athayde, G. P. B., de Castro, E. V. R., da Silva, F. L. F., et al. (2015). Evaluation and determination of chloride in crude oil based on the counterions Na, Ca, Mg, Sr and Fe, quantified via ICP-OES in the crude oil aqueous extract. Fuel, 154, 181–187. https://doi.org/10.1016/J.FUEL.2015.03.079.
Divjak, B., Franko, M., & Novič, M. (1998). Determination of iron in complex matrices by ion chromatography with UV–vis, thermal lens and amperometric detection using post-column reagents. Journal of Chromatography A, 829(1–2), 167–174. https://doi.org/10.1016/S0021-9673(98)00837-1.
dos Santos, A. B., Kohlmeier, K. A., Rocha, M. E., Barreto, G. E., Barreto, J. A., de Souza, A. C. A., & Bezerra, M. A. (2018). Hair in Parkinson’s disease patients exhibits differences in calcium, iron and zinc concentrations measured by flame atomic absorption spectrometry − FAAS. Journal of Trace Elements in Medicine and Biology, 47, 134–139. https://doi.org/10.1016/j.jtemb.2018.02.003.
Du, Y., Schuur, B., Kersten, S. R. A., & Brilman, D. W. F. (2015). Opportunities for switchable solvents for lipid extraction from wet algal biomass: An energy evaluation. Algal Research, 11, 271–283. https://doi.org/10.1016/J.ALGAL.2015.07.004.
Durukan, İ., Şahin, Ç. A., Şatıroğlu, N., & Bektaş, S. (2011). Determination of iron and copper in food samples by flow injection cloud point extraction flame atomic absorption spectrometry. Microchemical Journal. https://doi.org/10.1016/j.microc.2011.04.016.
Hashemi, B., Zohrabi, P., Kim, K.-H., Shamsipur, M., Deep, A., & Hong, J. (2017). Recent advances in liquid-phase microextraction techniques for the analysis of environmental pollutants. TrAC Trends in Analytical Chemistry, 97, 83–95. https://doi.org/10.1016/J.TRAC.2017.08.014.
Herrero, M., Mendiola, J. A., & Ibáñez, E. (2017). Gas expanded liquids and switchable solvents. Current Opinion in Green and Sustainable Chemistry, 5, 24–30. https://doi.org/10.1016/J.COGSC.2017.03.008.
Jeevan Kumar, S. P., Vijay Kumar, G., Dash, A., Scholz, P., & Banerjee, R. (2017). Sustainable green solvents and techniques for lipid extraction from microalgae: A review. Algal Research, 21, 138–147. https://doi.org/10.1016/J.ALGAL.2016.11.014.
Jessop, P. G., Phan, L., Carrier, A., Robinson, S., Christoph, J. D., & Harjani, J. R. (2010). A solvent having switchable hydrophilicity †, 809–814. https://doi.org/10.1039/b926885e.
Kasa, N. A., & Bakırdere, E. G. (n.d.). Determination of iron in liquorice samples by slotted quartz tube flame atomic absorption spectrometry with matrix matching calibration strategy after complexation with Schiff Base ligand based dispersive liquid-liquid microextraction.
Lasarte-aragonés, G., Lucena, R., Cárdenas, S., & Valcárcel, M. (2015). Talanta use of switchable solvents in the microextraction context. Talanta, 131, 645–649. https://doi.org/10.1016/j.talanta.2014.08.031.
Liu, X., & Hamon, J. R. (2019). Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coordination Chemistry Reviews. https://doi.org/10.1016/j.ccr.2019.03.010.
Liu, C.-G., Qiu, Y.-Q., Sun, S.-L., Li, N., Yang, G.-C., & Su, Z.-M. (2007). DFT studies on second-order nonlinear optical properties of mono (salicylaldiminato) nickel(II) polyenyl Schiff base metal complexes. Chemical Physics Letters, 443(1–3), 163–168. https://doi.org/10.1016/J.CPLETT.2007.06.060.
Matsumiya, H., Kato, T., & Hiraide, M. (2014). Ionic liquid-based extraction followed by graphite-furnace atomic absorption spectrometry for the determination of trace heavy metals in high-purity iron metal. Talanta, 119, 505–508. https://doi.org/10.1016/J.TALANTA.2013.11.057.
Moghadam, M. R., Shabani, A. M. H., & Dadfarnia, S. (2011). Spectrophotometric determination of iron species using a combination of artificial neural networks and dispersive liquid–liquid microextraction based on solidification of floating organic drop. Journal of Hazardous Materials, 197, 176–182. https://doi.org/10.1016/J.JHAZMAT.2011.09.073.
Naeemullah, & Tuzen, M. (2019). A new robust, deep eutectic-based floating organic droplets microextraction method for determination of lead in a portable syringe system directly couple with FAAS. Talanta, 196, 71–77. https://doi.org/10.1016/J.TALANTA.2018.12.027.
Peñalver, R., Campillo, N., López-García, I., & Hernández-Córdoba, M. (2020). Solid-phase microextraction for the determination of iron organic compounds in seawaters and soils by gas chromatography coupled to microwave-induced plasma with atomic emission detection spectrometry. Microchemical Journal, 154, 104630. https://doi.org/10.1016/J.MICROC.2020.104630.
Peng, B., Shen, Y., Gao, Z., Zhou, M., Ma, Y., & Zhao, S. (2015). Determination of total iron in water and foods by dispersive liquid–liquid microextraction coupled with microvolume UV–vis spectrophotometry. Food Chemistry, 176, 288–293. https://doi.org/10.1016/J.FOODCHEM.2014.12.084.
Reclo, M., Yilmaz, E., Soylak, M., Andruch, V., & Bazel, Y. (2017). Ligandless switchable solvent based liquid phase microextraction of nickel from food and cigarette samples prior to its micro-sampling flame atomic absorption spectrometric determination. Journal of Molecular Liquids. https://doi.org/10.1016/j.molliq.2017.04.066.
Rykowska, I., Ziemblińska, J., & Nowak, I. (2018). Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. Journal of Molecular Liquids, 259, 319–339. https://doi.org/10.1016/J.MOLLIQ.2018.03.043.
Shahvandi, S. K., Banitaba, M. H., Ahmar, H., & Karimi, P. (2020). A novel temperature controlled switchable solvent based microextraction method: Application for the determination of phthalic acid esters in water samples. Microchemical Journal, 152(August 2019), 104300. https://doi.org/10.1016/j.microc.2019.104300.
Soylak, M., Khan, M., & Yilmaz, E. (2016). Switchable solvent based liquid phase microextraction of uranium in environmental samples: A green approach. Analytical Methods, 8(5), 979–986.
Tabrizi, A. B. (2010). Development of a dispersive liquid–liquid microextraction method for iron speciation and determination in different water samples. Journal of Hazardous Materials, 183(1–3), 688–693. https://doi.org/10.1016/J.JHAZMAT.2010.07.080.
Yilmaz, E., & Soylak, M. (2015). Ultrasound assisted-deep eutectic solvent extraction of iron from sheep, bovine and chicken liver samples. Talanta, 136, 170–173. https://doi.org/10.1016/J.TALANTA.2014.12.034.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kasa, N.A., Bakirdere, E.G. & Bakirdere, S. A Simple and Green Vortex-Assisted Switchable Solvent-Based Microextraction Method by Using Schiff Base Ligand Complexation for Iron Determination in Mineral Spring Water Samples Prior to Slotted Quartz Tube Flame Atomic Absorption Spectrophotometry. Water Air Soil Pollut 231, 417 (2020). https://doi.org/10.1007/s11270-020-04754-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-020-04754-0