Abstract
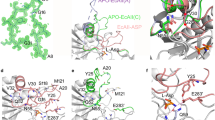
The apo form of the double mutant of Wolinella succinogenes L-asparaginase (WAS) with V23Q and K24T substitutions in the flexible N-terminal loop (WASm), which exhibits an order of magnitude lower glutaminase activity compared to the wild-type enzyme, was crystallized in two modifications (sp. grs. P22121 and P21). The three-dimensional structure in two modifications was determined at 1.5 and 1.7 Å resolution, respectively. The three-dimensional structures and the molecular packing modes of the enzyme in two crystal modifications (monoclinic, sp. gr. P21, and orthorhombic, sp. gr. P22121) are compared. Intermolecular contacts and solvent channels in both crystal lattices are described. The orthorhombic crystals have a closer packing compared to the monoclinic crystals and lower water content (36.95 and 44.53%, respectively). However, the active sites in both structures are solvent accessible.




Similar content being viewed by others
REFERENCES
J. B. Howard and F. J. Carpenter, J. Biol. Chem. 217, 1020 (1972).
J. Lubkowski, A. Wlodawer, H. L. Ammon, et al., Biochemistry 33, 10257 (1994).
J. Lubkowski, A. Wlodawer, D. Housset, et al., Acta Crystallogr. D 50, 826 (1994).
J. D. Broom, J. Exp. Med. 127, 1055 (1968).
K. H. Rohm and R. L. Van Etten, Arch. Biochem. Biophys. 244, 128 (1986).
J. H. Parmentier, M. Maggi, E. Tarasco, et al., Leuk. Res. 39, 757 (2015).
R. P. Warrell, T. C. Chou, C. Gordon, et al., Cancer Res. 40, 4546 (1980).
N. Patel, S. Krishnan, M. N. Offman, et al., J. Din. Invest. 119, 1964 (2009).
G. A. Kotzia, K. Lappa, and N. E. Labrou, Biochem. J. 404, 337 (2007).
M. N. Offman, M. Krol, N. Patel, et al., Blood 117, 1614 (2011).
J. A. Distasio and R. A. Niederman, J. Biol. Chem. 251, 6929 (1976).
J. A. Distasio, R. A. Niederman, and D. Kafkewitz, Exp. Biol. Med. 155, 528 (1977).
J. A. Distasio, A. M. Salazar, M. Nadji, and D. L. Durden, Int. J. Cancer 30, 343 (1982).
J. Lubkowski, G. J. Palm, G. L. Gilliland, et al., Eur. J. Biochem. 241, 201 (1996).
R. B. Reinert, L. M. Oberle, S. A. Wek, et al., J. Biol. Chem. 281, 31222 (2006).
D. L. Durden and J. A. Distasio, Cancer Res. 40, 1125 (1980).
D. L. Durden and J. A. Distasio, Int. J. Cancer 27, 59 (1981).
H. van den Berg, Leuk. Lymphoma 52, 168 (2011).
D. Covini, S. Tardito, O. Bussolati, et al., Recent Pat. Anti-Cancer Drug Discovery 7, 4 (2012).
A. Shrivastava, A. A. Khan, M. Khurshid, et al., Critical Rev. Oncol./Hematol. 100, 1 (2015).
E. P. Sannikova, N. V. Cheperegin. S. E. Bulushova, et al., Mol. Biotechnol. 58, 528 (2016). https://doi.org/10.1007/s12033
V. I. Timofeev, N. E. Zhukhlistova, and I. P. Kuranova, Bioorgan. Khim. 46, 140 (2020).
V. I. Timofeev, N. V. Bulushova, N. E. Zhukhlistova, and I. P. Kuranova, Crystallogr. Rep. 64 (6), 910 (2019).
A. J. McCoy, R. W. Grosse-Kunstleve, P. D. Adams, et al., J. Appl. Crystallogr. 40, 658 (2007).
G. N. Murshudov, A. A. Vagin, and E. J. Dodson, Acta Crystallogr. D 53, 240 (1997).
P. Emsley and K. Cowtan, Acta Crystallogr. D 60, 2126 (2004).
Collaborative Computational Project No. 4 “The CCP4 Suite: Programs for Protein Crystallography,” Acta Crystallogr. D 50, 760 (1994).
E. Krissinel and K. Henrick, J. Mol. Biol. 372, 774 (2007).
L. L. C. Schrödinger, The PyMOL Molecular Graphics System, Version 1.8 (2015).
B. W. Matthews, J. Mol. Biol. 33, 491 (1968).
Funding
The study was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the state assignment of the Federal Scientific Research Centre “Crystallography and Photonics” of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by T. Safonova
Rights and permissions
About this article
Cite this article
Timofeev, V.I., Zhukhlistova, N.E. & Kuranova, I.P. Molecular Packing of a Mutant of L-Asparaginase from Wolinella succinigenes in Two Crystal Modifications. Crystallogr. Rep. 65, 586–592 (2020). https://doi.org/10.1134/S1063774520040227
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063774520040227